Extremely low exposure of a community to severe acute respiratory syndrome coronavirus: false seropositivity due to use of bacterially derived antigens
- PMID: 16940504
- PMCID: PMC1563915
- DOI: 10.1128/JVI.00649-06
Extremely low exposure of a community to severe acute respiratory syndrome coronavirus: false seropositivity due to use of bacterially derived antigens
Abstract
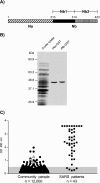
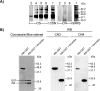
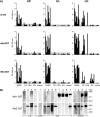
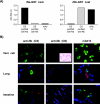
Estimates of seropositivity to a new infectious agent in a community are useful to public health. For severe acute respiratory syndrome (SARS), the figures are conflicting. Herein, we screened 12,000 people in a community stricken by SARS 10 months previously and found 53 individuals (0.44%) who had immunoglobulin G antibodies to the SARS coronavirus (SARS-CoV) nucleocapsid (N) produced in bacteria. However, only seven of these (group 1) had sera which also reacted with the native N antigen expressed in SARS-CoV-infected Vero cells, N-transfected 293T cells, and tissues of infected SARS patients. Of these, six individuals had had SARS previously. The remaining person, as well as the 46 other individuals (group 2), were healthy and had no history of SARS. Group 1 antibodies recognized epitopes located slightly differently in N from those of group 2 antibodies, and a mouse hybridoma antibody resembling the former type was generated. Unusually, group 2 antibodies appeared to recognize cross-reactive bacterial epitopes that presumably were posttranslationally modified in eukaryotes and hence were probably not induced by SARS-CoV or related coronaviruses but rather by bacteria. The N antigen is thus highly unique. The extremely low rate (0.008%) of asymptomatic SARS infection found attests to the high virulence of the SARS-CoV virus.
Figures

Similar articles
-
Biochemical and immunological studies of nucleocapsid proteins of severe acute respiratory syndrome and 229E human coronaviruses.Proteomics. 2005 Mar;5(4):925-37. doi: 10.1002/pmic.200401204. Proteomics. 2005. PMID: 15759315 Free PMC article.
-
Characterization and epitope mapping of monoclonal antibodies to the nucleocapsid protein of severe acute respiratory syndrome coronavirus.Jpn J Vet Res. 2008 Feb;55(4):115-27. Jpn J Vet Res. 2008. PMID: 18380153
-
Antibody-dependent SARS coronavirus infection is mediated by antibodies against spike proteins.Biochem Biophys Res Commun. 2014 Aug 22;451(2):208-14. doi: 10.1016/j.bbrc.2014.07.090. Epub 2014 Jul 26. Biochem Biophys Res Commun. 2014. PMID: 25073113 Free PMC article.
-
Detection of specific antibodies to severe acute respiratory syndrome (SARS) coronavirus nucleocapsid protein for serodiagnosis of SARS coronavirus pneumonia.J Clin Microbiol. 2004 May;42(5):2306-9. doi: 10.1128/JCM.42.5.2306-2309.2004. J Clin Microbiol. 2004. PMID: 15131220 Free PMC article.
-
Severe acute respiratory syndrome vaccine development: experiences of vaccination against avian infectious bronchitis coronavirus.Avian Pathol. 2003 Dec;32(6):567-82. doi: 10.1080/03079450310001621198. Avian Pathol. 2003. PMID: 14676007 Free PMC article. Review.
Cited by
-
The SARS-CoV nucleocapsid protein: a protein with multifarious activities.Infect Genet Evol. 2008 Jul;8(4):397-405. doi: 10.1016/j.meegid.2007.07.004. Epub 2007 Jul 20. Infect Genet Evol. 2008. PMID: 17881296 Free PMC article. Review.
-
Recombinant viral proteins for use in diagnostic ELISAs to detect virus infection.Vaccine. 2007 Jul 26;25(30):5653-9. doi: 10.1016/j.vaccine.2007.02.053. Epub 2007 Mar 6. Vaccine. 2007. PMID: 17478017 Free PMC article. Review.
-
Coronaviruses post-SARS: update on replication and pathogenesis.Nat Rev Microbiol. 2009 Jun;7(6):439-50. doi: 10.1038/nrmicro2147. Nat Rev Microbiol. 2009. PMID: 19430490 Free PMC article. Review.
-
ADE and hyperinflammation in SARS-CoV2 infection- comparison with dengue hemorrhagic fever and feline infectious peritonitis.Cytokine. 2020 Dec;136:155256. doi: 10.1016/j.cyto.2020.155256. Epub 2020 Aug 20. Cytokine. 2020. PMID: 32866898 Free PMC article. Review.
-
Antigen Production in Plant to Tackle Infectious Diseases Flare Up: The Case of SARS.Front Plant Sci. 2016 Feb 5;7:54. doi: 10.3389/fpls.2016.00054. eCollection 2016. Front Plant Sci. 2016. PMID: 26904039 Free PMC article.
References
-
- Chan, V. S., K. Y. Chan, Y. Chen, L. L. Poon, A. N. Cheung, B. Zheng, K. H. Chan, W. Mak, H. Y. Ngan, X. Xu, G. Screaton, P. K. Tam, J. M. Austyn, L. C. Chan, S. P. Yip, M. Peiris, U. S. Khoo, and C. L. Lin. 2006. Homozygous L-SIGN (CLEC4M) plays a protective role in SARS coronavirus infection. Nat. Genet. 38:38-46. - PMC - PubMed
-
- Che, X. Y., L. W. Qiu, Y. X. Pan, K. Wen, W. Hao, L. Y. Zhang, Y. D. Wang, Z. Y. Liao, X. Hua, V. C. Cheng, and K. Y. Yuen. 2004. Sensitive and specific monoclonal antibody-based capture enzyme immunoassay for detection of nucleocapsid antigen in sera from patients with severe acute respiratory syndrome. J. Clin. Microbiol. 42:2629-2635. - PMC - PubMed
-
- Chien, Y. C., J. Y. Chen, M. Y. Liu, H. I. Yang, M. M. Hsu, C. J. Chen, and C. S. Yang. 2001. Serologic markers of Epstein-Barr virus infection and nasopharyngeal carcinoma in Taiwanese men. N. Engl. J. Med. 345:1877-1882. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

