Tcof1/Treacle is required for neural crest cell formation and proliferation deficiencies that cause craniofacial abnormalities
- PMID: 16938878
- PMCID: PMC1557391
- DOI: 10.1073/pnas.0603730103
Tcof1/Treacle is required for neural crest cell formation and proliferation deficiencies that cause craniofacial abnormalities
Abstract
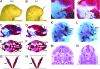
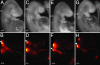
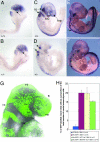
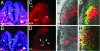
Neural crest cells are a migratory cell population that give rise to the majority of the cartilage, bone, connective tissue, and sensory ganglia in the head. Abnormalities in the formation, proliferation, migration, and differentiation phases of the neural crest cell life cycle can lead to craniofacial malformations, which constitute one-third of all congenital birth defects. Treacher Collins syndrome (TCS) is characterized by hypoplasia of the facial bones, cleft palate, and middle and external ear defects. Although TCS results from autosomal dominant mutations of the gene TCOF1, the mechanistic origins of the abnormalities observed in this condition are unknown, and the function of Treacle, the protein encoded by TCOF1, remains poorly understood. To investigate the developmental basis of TCS we generated a mouse model through germ-line mutation of Tcof1. Haploinsufficiency of Tcof1 leads to a deficiency in migrating neural crest cells, which results in severe craniofacial malformations. We demonstrate that Tcof1/Treacle is required cell-autonomously for the formation and proliferation of neural crest cells. Tcof1/Treacle regulates proliferation by controlling the production of mature ribosomes. Therefore, Tcof1/Treacle is a unique spatiotemporal regulator of ribosome biogenesis, a deficiency that disrupts neural crest cell formation and proliferation, causing the hypoplasia characteristic of TCS craniofacial anomalies.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures

Similar articles
-
Face off against ROS: Tcof1/Treacle safeguards neuroepithelial cells and progenitor neural crest cells from oxidative stress during craniofacial development.Dev Growth Differ. 2016 Sep;58(7):577-85. doi: 10.1111/dgd.12305. Epub 2016 Aug 2. Dev Growth Differ. 2016. PMID: 27481486 Free PMC article. Review.
-
Treacher Collins syndrome: unmasking the role of Tcof1/treacle.Int J Biochem Cell Biol. 2009 Jun;41(6):1229-32. doi: 10.1016/j.biocel.2008.10.026. Epub 2008 Nov 5. Int J Biochem Cell Biol. 2009. PMID: 19027870 Free PMC article. Review.
-
Treacher Collins syndrome.Orthod Craniofac Res. 2007 May;10(2):88-95. doi: 10.1111/j.1601-6343.2007.00388.x. Orthod Craniofac Res. 2007. PMID: 17552945 Review.
-
Increased levels of apoptosis in the prefusion neural folds underlie the craniofacial disorder, Treacher Collins syndrome.Hum Mol Genet. 2000 Jun 12;9(10):1473-80. doi: 10.1093/hmg/9.10.1473. Hum Mol Genet. 2000. PMID: 10888597
-
Genetic background has a major effect on the penetrance and severity of craniofacial defects in mice heterozygous for the gene encoding the nucleolar protein Treacle.Dev Dyn. 2004 Apr;229(4):907-14. doi: 10.1002/dvdy.20004. Dev Dyn. 2004. PMID: 15042714
Cited by
-
Re-focusing on Agnathia-Otocephaly complex.Clin Oral Investig. 2021 Mar;25(3):1353-1362. doi: 10.1007/s00784-020-03443-w. Epub 2020 Jul 9. Clin Oral Investig. 2021. PMID: 32643087 Review.
-
Large deletions encompassing the TCOF1 and CAMK2A genes are responsible for Treacher Collins syndrome with intellectual disability.Eur J Hum Genet. 2014 Jan;22(1):52-6. doi: 10.1038/ejhg.2013.98. Epub 2013 May 22. Eur J Hum Genet. 2014. PMID: 23695276 Free PMC article.
-
A common cellular response to broad splicing perturbations is characterized by metabolic transcript downregulation driven by the Mdm2-p53 axis.Dis Model Mech. 2024 Feb 1;17(2):dmm050356. doi: 10.1242/dmm.050356. Epub 2024 Mar 1. Dis Model Mech. 2024. PMID: 38426258 Free PMC article.
-
When ribosomes go bad: diseases of ribosome biogenesis.Mol Biosyst. 2010 Mar;6(3):481-93. doi: 10.1039/b919670f. Epub 2010 Jan 11. Mol Biosyst. 2010. PMID: 20174677 Free PMC article. Review.
-
Facial dysostoses: Etiology, pathogenesis and management.Am J Med Genet C Semin Med Genet. 2013 Nov;163C(4):283-94. doi: 10.1002/ajmg.c.31375. Epub 2013 Oct 4. Am J Med Genet C Semin Med Genet. 2013. PMID: 24123981 Free PMC article. Review.
References
-
- Trainor P., Bronner-Fraser M., Krumlauf R. In: Handbook of Stem Cells: Embryonic Stem Cells. Lanza G., Weissman I., Thomson J., Pedersen R., Hogan B., Gearhart J., Blau H., Melton D., Moore M., Verfaillie C., et al., editors. Boston: Academic; 2004. pp. 219–232.
-
- Selleck M. A., Bronner-Fraser M. Development (Cambridge, U.K.) 1995;121:525–538. - PubMed
-
- Bronner-Fraser M., Fraser S. Neuron. 1989;3:755–766. - PubMed
-
- Moury J. D., Jacobson A. G. Dev. Biol. 1990;141:243–253. - PubMed
-
- Rollhauser-ter Horst J. Anat. Embryol. 1977;151:309–316. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

