B and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal disease
- PMID: 16936272
- PMCID: PMC1698808
- DOI: 10.2353/ajpath.2006.060180
B and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal disease
Abstract
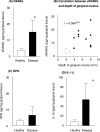
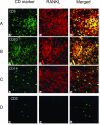
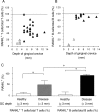
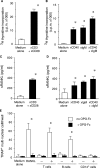
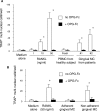
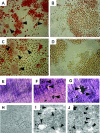
Receptor activator of nuclear factor-kappaB (RANKL)-mediated osteoclastogenesis plays a pivotal role in inflammatory bone resorption. The aim of this study was to identify the cellular source of RANKL in the bone resorptive lesions of periodontal disease. The concentrations of soluble RANKL, but not its decoy receptor osteoprotegerin, measured in diseased tissue homogenates were significantly higher in diseased gingival tissues than in healthy tissues. Double-color confocal microscopic analyses demonstrated less than 20% of both B cells and T cells expressing RANKL in healthy gingival tissues. By contrast, in the abundant mononuclear cells composed of 45% T cells, 50% B cells, and 5% monocytes in diseased gingival tissues, more than 50 and 90% of T cells and B cells, respectively, expressed RANKL. RANKL production by nonlymphoid cells was not distinctly identified. Lymphocytes isolated from gingival tissues of patients induced differentiation of mature osteoclast cells in a RANKL-dependent manner in vitro. However, similarly isolated peripheral blood B and T cells did not induce osteoclast differentiation, unless they were activated in vitro to express RANKL; emphasizing the osteoclastogenic potential of activated RANKL-expressing lymphocytes in periodontal disease tissue. These results suggest that activated T and B cells can be the cellular source of RANKL for bone resorption in periodontal diseased gingival tissue.
Figures

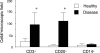
Similar articles
-
Immune response: the key to bone resorption in periodontal disease.J Periodontol. 2005 Nov;76(11 Suppl):2033-41. doi: 10.1902/jop.2005.76.11-S.2033. J Periodontol. 2005. PMID: 16277573 Review.
-
Soluble RANKL Cleaved from Activated Lymphocytes by TNF-α-Converting Enzyme Contributes to Osteoclastogenesis in Periodontitis.J Immunol. 2016 Nov 15;197(10):3871-3883. doi: 10.4049/jimmunol.1601114. Epub 2016 Oct 7. J Immunol. 2016. PMID: 27815441 Free PMC article.
-
LPS-stimulated human gingival fibroblasts inhibit the differentiation of monocytes into osteoclasts through the production of osteoprotegerin.Clin Exp Immunol. 2002 Nov;130(2):338-44. doi: 10.1046/j.1365-2249.2002.01990.x. Clin Exp Immunol. 2002. PMID: 12390325 Free PMC article.
-
Selective blockade of voltage-gated potassium channels reduces inflammatory bone resorption in experimental periodontal disease.J Bone Miner Res. 2004 Jan;19(1):155-64. doi: 10.1359/JBMR.0301213. J Bone Miner Res. 2004. PMID: 14753747
-
Role of receptor activator of nuclear factor-kappaB ligand and osteoprotegerin in bone cell biology.J Mol Med (Berl). 2001 Jun;79(5-6):243-53. doi: 10.1007/s001090100226. J Mol Med (Berl). 2001. PMID: 11485016 Review.
Cited by
-
RANKL and osteoimmunology in periodontitis.J Bone Miner Metab. 2021 Jan;39(1):82-90. doi: 10.1007/s00774-020-01165-3. Epub 2020 Oct 17. J Bone Miner Metab. 2021. PMID: 33070252 Review.
-
Transcriptome Analysis of B Cell Immune Functions in Periodontitis: Mucosal Tissue Responses to the Oral Microbiome in Aging.Front Immunol. 2016 Jul 18;7:272. doi: 10.3389/fimmu.2016.00272. eCollection 2016. Front Immunol. 2016. PMID: 27486459 Free PMC article.
-
Constitutive expression of TNF-related activation-induced cytokine (TRANCE)/receptor activating NF-κB ligand (RANK)-L by rat plasmacytoid dendritic cells.PLoS One. 2012;7(3):e33713. doi: 10.1371/journal.pone.0033713. Epub 2012 Mar 13. PLoS One. 2012. PMID: 22428075 Free PMC article.
-
T and B Cells in Periodontal Disease: New Functions in A Complex Scenario.Int J Mol Sci. 2019 Aug 14;20(16):3949. doi: 10.3390/ijms20163949. Int J Mol Sci. 2019. PMID: 31416146 Free PMC article. Review.
-
Pyrroloquinoline quinone inhibits ligature-induced alveolar bone loss through regulation of redox balance and cell senescence.Am J Transl Res. 2022 Jan 15;14(1):582-593. eCollection 2022. Am J Transl Res. 2022. PMID: 35173876 Free PMC article.
References
-
- McArthur WP, Clark WB. Specific antibodies and their potential role in periodontal diseases. J Periodontol. 1993;64:807–818. - PubMed
-
- Taubman MA, Ebersole JL, Smith DJ. Genco RJ, Mergenhangen SE, editors. Washington DC: American Society of Microbiology,; Association between systemic and local antibody and periodontal diseases. Host Parasite Interactions in Periodontal Diseases. 1982:pp 283–298.
-
- Ebersole JL, Taubman MA, Smith DJ, Frey DE, Haffajee AD, Socransky SS. Human serum antibody responses to oral microorganisms. IV. Correlation with homologous infection. Oral Microbiol Immunol. 1987;2:53–59. - PubMed
-
- Wu X, Pan G, McKenna MA, Zayzafoon M, Xiong WC, McDonald JM. RANKL regulates Fas expression and Fas-mediated apoptosis in osteoclasts. J Bone Miner Res. 2005;20:107–116. - PubMed
-
- Hofbauer LC, Khosla S, Dunstan CR, Lacey DL, Boyle WJ, Riggs BL. The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J Bone Miner Res. 2000;15:2–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

