Cellular senescence in naevi and immortalisation in melanoma: a role for p16?
- PMID: 16880792
- PMCID: PMC2360676
- DOI: 10.1038/sj.bjc.6603283
Cellular senescence in naevi and immortalisation in melanoma: a role for p16?
Abstract
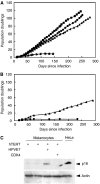
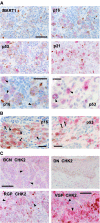
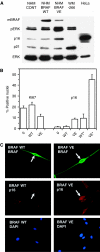
Cellular senescence, the irreversible proliferative arrest seen in somatic cells after a limited number of divisions, is considered a crucial barrier to cancer, but direct evidence for this in vivo was lacking until recently. The best-known form of human cell senescence is attributed to telomere shortening and a DNA-damage response through p53 and p21. There is also a more rapid form of senescence, dependent on the p16-retinoblastoma pathway. p16 (CDKN2A) is a known melanoma susceptibility gene. Here, we use retrovirally mediated gene transfer to confirm that the normal form of senescence in cultured human melanocytes involves p16, since disruption of the p16/retinoblastoma pathway is required as well as telomerase activation for immortalisation. Expression (immunostaining) patterns of senescence mediators and markers in melanocytic lesions provide strong evidence that cell senescence occurs in benign melanocytic naevi (moles) in vivo and does not involve p53 or p21 upregulation, although p16 is widely expressed. In comparison, dysplastic naevi and early (radial growth-phase, RGP) melanomas show less p16 and some p53 and p21 immunostaining. All RGP melanomas expressed p21, suggesting areas of p53-mediated senescence, while most areas of advanced (vertical growth-phase) melanomas lacked both p16 and p21, implying escape from both forms of senescence (immortalisation). Moreover, nuclear p16 but not p21 expression can be induced in human melanocytes by oncogenic BRAF, as found in around 80% of naevi. We conclude that cell senescence can form a barrier to melanoma development. This also provides a potential explanation of why p16 is a melanoma suppressor gene.
Figures


Similar articles
-
The relative contributions of the p53 and pRb pathways in oncogene-induced melanocyte senescence.Aging (Albany NY). 2009 May 16;1(6):542-56. doi: 10.18632/aging.100051. Aging (Albany NY). 2009. PMID: 20157537 Free PMC article.
-
Melanocytic nevus-like hyperplasia and melanoma in transgenic BRAFV600E mice.Oncogene. 2009 Jun 11;28(23):2289-98. doi: 10.1038/onc.2009.95. Epub 2009 Apr 27. Oncogene. 2009. PMID: 19398955 Free PMC article.
-
Senescence evasion in melanoma progression: uncoupling of DNA-damage signaling from p53 activation and p21 expression.Pigment Cell Melanoma Res. 2013 Mar;26(2):226-35. doi: 10.1111/pcmr.12060. Epub 2013 Jan 14. Pigment Cell Melanoma Res. 2013. PMID: 23253087 Free PMC article.
-
Melanomagenesis: overcoming the barrier of melanocyte senescence.Cell Cycle. 2008 Jul 1;7(13):1944-8. doi: 10.4161/cc.7.13.6230. Epub 2008 Apr 23. Cell Cycle. 2008. PMID: 18604170 Free PMC article. Review.
-
Cellular senescence and tumor suppressor gene p16.Int J Cancer. 2012 Apr 15;130(8):1715-25. doi: 10.1002/ijc.27316. Epub 2011 Dec 5. Int J Cancer. 2012. PMID: 22025288 Free PMC article. Review.
Cited by
-
Coexpression of SOX10/CD271 (p75(NTR)) and β-Galactosidase in Large to Giant Congenital Melanocytic Nevi of Pediatric Patients.Dermatopathology (Basel). 2014 May 1;1(1):35-46. doi: 10.1159/000362490. eCollection 2014 Jan-Jul. Dermatopathology (Basel). 2014. PMID: 27047921 Free PMC article.
-
ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases.Oxid Med Cell Longev. 2016;2016:3565127. doi: 10.1155/2016/3565127. Epub 2016 May 10. Oxid Med Cell Longev. 2016. PMID: 27247702 Free PMC article. Review.
-
The paradox of senescent-marker positive cancer cells: challenges and opportunities.NPJ Aging. 2024 Sep 14;10(1):41. doi: 10.1038/s41514-024-00168-y. NPJ Aging. 2024. PMID: 39277623 Free PMC article. Review.
-
The dysplastic nevus: from historical perspective to management in the modern era: part II. Molecular aspects and clinical management.J Am Acad Dermatol. 2012 Jul;67(1):19.e1-12; quiz 31-2. doi: 10.1016/j.jaad.2012.03.013. J Am Acad Dermatol. 2012. PMID: 22703916 Free PMC article.
-
Biomarkers of Cellular Senescence and Skin Aging.Front Genet. 2018 Aug 23;9:247. doi: 10.3389/fgene.2018.00247. eCollection 2018. Front Genet. 2018. PMID: 30190724 Free PMC article. Review.
References
-
- Bandyopadhyay D, Timchenko N, Suwa T, Hornsby PJ, Campisi J, Medrano EE (2001) The human melanocyte: a model system to study the complexity of cellular aging and transformation in non-fibroblastic cells. Exp Gerontol 36: 1265–1275 - PubMed
-
- Bartkova J, Lukas J, Guldberg P, Alsner J, Kirkin AF, Zeuthen J, Bartek J (1996) The p16-cyclin D/Cdk4-pRb pathway as a functional unit frequently altered in melanoma pathogenesis. Cancer Res 56: 5475–5483 - PubMed
-
- Bastian BC (2003) The longer your telomeres, the larger your nevus? Am J Dermatopathol 25: 83–84 - PubMed
-
- Bennett DC (2003) Human melanocyte senescence and melanoma susceptibility genes. Oncogene 22: 3063–3069 - PubMed
-
- Bennett DC (2006) Familial melanoma genes, melanocyte immortalization and melanoma initiation. In: Melanocytes to Melanoma: The Progression to Malignancy, Hearing VJ, Leong SPL (eds) New Jersey: Humana Press
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

