Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons
- PMID: 16874299
- PMCID: PMC1553198
- DOI: 10.1038/sj.emboj.7601253
Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons
Abstract
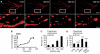
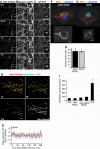
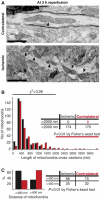
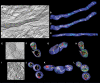
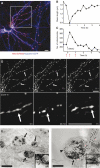
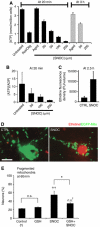
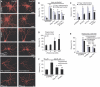
Mitochondria are present as tubular organelles in neuronal projections. Here, we report that mitochondria undergo profound fission in response to nitric oxide (NO) in cortical neurons of primary cultures. Mitochondrial fission by NO occurs long before neurite injury and neuronal cell death. Furthermore, fission is accompanied by ultrastructural damage of mitochondria, autophagy, ATP decline and generation of free radicals. Fission is occasionally asymmetric and can be reversible. Strikingly, mitochondrial fission is also an early event in ischemic stroke in vivo. Mitofusin 1 (Mfn1) or dominant-negative Dynamin related protein 1 (Drp1(K38A)) inhibits mitochondrial fission induced by NO, rotenone and Amyloid-beta peptide. Conversely, overexpression of Drp1 or Fis1 elicits fission and increases neuronal loss. Importantly, NO-induced neuronal cell death was mitigated by Mfn1 and Drp1(K38A). Thus, persistent mitochondrial fission may play a causal role in NO-mediated neurotoxicity.
Figures
Similar articles
-
Mitochondrial fission is an upstream and required event for bax foci formation in response to nitric oxide in cortical neurons.Cell Death Differ. 2007 Mar;14(3):462-71. doi: 10.1038/sj.cdd.4402046. Epub 2006 Oct 20. Cell Death Differ. 2007. PMID: 17053808
-
Aβ-Induced Drp1 phosphorylation through Akt activation promotes excessive mitochondrial fission leading to neuronal apoptosis.Biochim Biophys Acta. 2016 Nov;1863(11):2820-2834. doi: 10.1016/j.bbamcr.2016.09.003. Epub 2016 Sep 4. Biochim Biophys Acta. 2016. PMID: 27599716
-
Impaired balance of mitochondrial fission and fusion in Alzheimer's disease.J Neurosci. 2009 Jul 15;29(28):9090-103. doi: 10.1523/JNEUROSCI.1357-09.2009. J Neurosci. 2009. PMID: 19605646 Free PMC article.
-
Dynamin-related protein 1 and mitochondrial fragmentation in neurodegenerative diseases.Brain Res Rev. 2011 Jun 24;67(1-2):103-18. doi: 10.1016/j.brainresrev.2010.11.004. Epub 2010 Dec 8. Brain Res Rev. 2011. PMID: 21145355 Free PMC article. Review.
-
[Mechanism of mitochondrial fission - structure and function of Drp1 protein].Postepy Biochem. 2016;62(2):127-137. Postepy Biochem. 2016. PMID: 28132464 Review. Polish.
Cited by
-
Implications of mitochondrial dynamics on neurodegeneration and on hypothalamic dysfunction.Front Aging Neurosci. 2015 Jun 10;7:101. doi: 10.3389/fnagi.2015.00101. eCollection 2015. Front Aging Neurosci. 2015. PMID: 26113818 Free PMC article. Review.
-
Lysocardiolipin acyltransferase 1 (ALCAT1) controls mitochondrial DNA fidelity and biogenesis through modulation of MFN2 expression.Proc Natl Acad Sci U S A. 2012 May 1;109(18):6975-80. doi: 10.1073/pnas.1120043109. Epub 2012 Apr 16. Proc Natl Acad Sci U S A. 2012. PMID: 22509026 Free PMC article.
-
Cellular alterations in human traumatic brain injury: changes in mitochondrial morphology reflect regional levels of injury severity.J Neurotrauma. 2013 Mar 1;30(5):367-81. doi: 10.1089/neu.2012.2339. J Neurotrauma. 2013. PMID: 23131111 Free PMC article.
-
Protective effects of reduced dynamin-related protein 1 against amyloid beta-induced mitochondrial dysfunction and synaptic damage in Alzheimer's disease.Hum Mol Genet. 2016 Dec 1;25(23):5148-5166. doi: 10.1093/hmg/ddw330. Hum Mol Genet. 2016. PMID: 27677309 Free PMC article.
-
PGC-1α, mitochondrial dysfunction, and Huntington's disease.Free Radic Biol Med. 2013 Sep;62:37-46. doi: 10.1016/j.freeradbiomed.2013.04.016. Epub 2013 Apr 19. Free Radic Biol Med. 2013. PMID: 23602910 Free PMC article. Review.
References
-
- Beal MF (2000) Energetics in the pathogenesis of neurodegenerative diseases. Trends Neurosci 23: 298–304 - PubMed
-
- Bereiter-Hahn J, Voth M (1994) Dynamics of mitochondria in living cells: shape changes, dislocations, fusion, and fission of mitochondria. Microsc Res Tech 27: 198–219 - PubMed
-
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT (2000) Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci 3: 1301–1306 - PubMed
-
- Boje KM (2004) Nitric oxide neurotoxicity in neurodegenerative diseases. Front Biosci 9: 763–776 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS044326/NS/NINDS NIH HHS/United States
- R01 EY009024/EY/NEI NIH HHS/United States
- R01 NS044314/NS/NINDS NIH HHS/United States
- P41RR04050/RR/NCRR NIH HHS/United States
- R01 EY016164/EY/NEI NIH HHS/United States
- R01 NS14718/NS/NINDS NIH HHS/United States
- R01 EY005477/EY/NEI NIH HHS/United States
- R01 NS43242/NS/NINDS NIH HHS/United States
- P41 RR004050/RR/NCRR NIH HHS/United States
- R01 EY05477/EY/NEI NIH HHS/United States
- R01 NS014718/NS/NINDS NIH HHS/United States
- R01 EY09024/EY/NEI NIH HHS/United States
- R01 NS44314/NS/NINDS NIH HHS/United States
- P01 AI055789/AI/NIAID NIH HHS/United States
- P01 HD029587/HD/NICHD NIH HHS/United States
- R01 NS047973/NS/NINDS NIH HHS/United States
- R01 NS043242/NS/NINDS NIH HHS/United States
- R01 NS047456/NS/NINDS NIH HHS/United States
- R01 NS046994/NS/NINDS NIH HHS/United States
- R01 NS047456-S3/NS/NINDS NIH HHS/United States
- P01 HD29587/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

