Calcineurin-dependent cardiomyopathy is activated by TRPC in the adult mouse heart
- PMID: 16873889
- PMCID: PMC2693319
- DOI: 10.1096/fj.05-5560com
Calcineurin-dependent cardiomyopathy is activated by TRPC in the adult mouse heart
Abstract
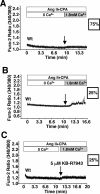
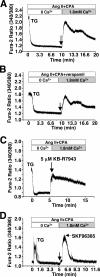
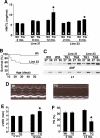
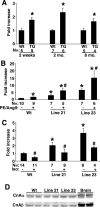
The manner in which Ca2+-sensitive signaling proteins are activated in contracting cardiomyocytes is an intriguing theoretical problem given that the cytoplasm is continually bathed with systolic Ca2+ concentrations that should maximally activate most Ca2+-sensitive signaling kinases and phosphatases. Store-operated Ca2+ entry, partially attributed to transient receptor potential (TRP) proteins, can mediate activation of the Ca2+-sensitive phosphatase calcineurin in nonexcitable cells. Here we investigated the gain-of-function phenotype associated with TRPC3 expression in the mouse heart using transgenesis to examine the potential role of store-operated Ca2+ entry in regulating cardiac calcineurin activation and ensuing hypertrophy/myopathy. Adult myocytes isolated from TRPC3 transgenic mice showed abundant store-operated Ca2+ entry that was inhibited with SKF96365 but not verapamil or KB-R7943. Associated with this induction in store-operated Ca2+ entry, TRPC3 transgenic mice showed increased calcineurin-nuclear factor of activated T cells (NFAT) activation in vivo, cardiomyopathy, and increased hypertrophy after neuroendocrine agonist or pressure overload stimulation. The cardiomyopathic phenotype and increased hypertrophy after pressure overload stimulation were blocked by targeted disruption of the calcineurin Abeta gene. Thus, enhanced store-operated Ca2+ entry in the heart can regulate calcineurin-NFAT signaling in vivo, which could secondarily impact the hypertrophic response and cardiomyopathy.
Figures

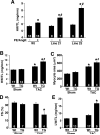
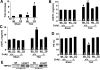
Similar articles
-
TRPC6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling.J Clin Invest. 2006 Dec;116(12):3114-26. doi: 10.1172/JCI27702. Epub 2006 Nov 9. J Clin Invest. 2006. PMID: 17099778 Free PMC article.
-
PKC-dependent coupling of calcium permeation through transient receptor potential canonical 3 (TRPC3) to calcineurin signaling in HL-1 myocytes.Proc Natl Acad Sci U S A. 2011 Jun 28;108(26):10556-61. doi: 10.1073/pnas.1106183108. Epub 2011 Jun 8. Proc Natl Acad Sci U S A. 2011. PMID: 21653882 Free PMC article.
-
TRPC channels are necessary mediators of pathologic cardiac hypertrophy.Proc Natl Acad Sci U S A. 2010 Apr 13;107(15):7000-5. doi: 10.1073/pnas.1001825107. Epub 2010 Mar 29. Proc Natl Acad Sci U S A. 2010. PMID: 20351294 Free PMC article.
-
TRPC channels as effectors of cardiac hypertrophy.Circ Res. 2011 Jan 21;108(2):265-72. doi: 10.1161/CIRCRESAHA.110.225888. Circ Res. 2011. PMID: 21252153 Review.
-
Cardiac Remodeling and Disease: SOCE and TRPC Signaling in Cardiac Pathology.Adv Exp Med Biol. 2017;993:505-521. doi: 10.1007/978-3-319-57732-6_25. Adv Exp Med Biol. 2017. PMID: 28900930 Review.
Cited by
-
SOCE in the cardiomyocyte: the secret is in the chambers.Pflugers Arch. 2021 Mar;473(3):417-434. doi: 10.1007/s00424-021-02540-3. Epub 2021 Feb 27. Pflugers Arch. 2021. PMID: 33638008 Free PMC article. Review.
-
The Ca(2+)-activated cation channel TRPM4 is a negative regulator of angiotensin II-induced cardiac hypertrophy.Basic Res Cardiol. 2015;110(4):43. doi: 10.1007/s00395-015-0501-x. Epub 2015 Jun 5. Basic Res Cardiol. 2015. PMID: 26043922 Free PMC article.
-
Increased size and cellularity of advanced atherosclerotic lesions in mice with endothelial overexpression of the human TRPC3 channel.Proc Natl Acad Sci U S A. 2015 Apr 28;112(17):E2201-6. doi: 10.1073/pnas.1505410112. Epub 2015 Apr 13. Proc Natl Acad Sci U S A. 2015. PMID: 25870279 Free PMC article.
-
A background Ca2+ entry pathway mediated by TRPC1/TRPC4 is critical for development of pathological cardiac remodelling.Eur Heart J. 2015 Sep 1;36(33):2257-66. doi: 10.1093/eurheartj/ehv250. Epub 2015 Jun 11. Eur Heart J. 2015. PMID: 26069213 Free PMC article.
-
Canonical TRP channels and mechanotransduction: from physiology to disease states.Pflugers Arch. 2010 Aug;460(3):571-81. doi: 10.1007/s00424-010-0847-8. Epub 2010 May 21. Pflugers Arch. 2010. PMID: 20490539 Review.
References
-
- Klee CB, Ren H, Wang X. Regulation of the calmodulin-stimulated protein phosphatase, calcineurin. J. Biol. Chem. 1998;273:13367–13370. - PubMed
-
- Graef IA, Chen F, Crabtree GR. NFAT signaling in vertebrate development. Curr. Opin. Genet. Dev. 2001;11:505–512. - PubMed
-
- Crabtree GR, Olson EN. NFAT signaling: choreographing the social lives of cells. Cell. 2002;109:S67–S79. - PubMed
-
- Hogan PG, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003;17:2205–2232. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

