Infection of human airway epithelium by human and avian strains of influenza a virus
- PMID: 16873262
- PMCID: PMC1563802
- DOI: 10.1128/JVI.00384-06
Infection of human airway epithelium by human and avian strains of influenza a virus
Abstract
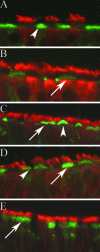
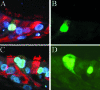
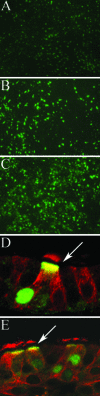
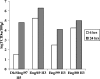
We describe the characterization of influenza A virus infection of an established in vitro model of human pseudostratified mucociliary airway epithelium (HAE). Sialic acid receptors for both human and avian viruses, alpha-2,6- and alpha-2,3-linked sialic acids, respectively, were detected on the HAE cell surface, and their distribution accurately reflected that in human tracheobronchial tissue. Nonciliated cells present a higher proportion of alpha-2,6-linked sialic acid, while ciliated cells possess both sialic acid linkages. Although we found that human influenza viruses infected both ciliated and nonciliated cell types in the first round of infection, recent human H3N2 viruses infected a higher proportion of nonciliated cells in HAE than a 1968 pandemic-era human virus, which infected proportionally more ciliated cells. In contrast, avian influenza viruses exclusively infected ciliated cells. Although a broad-range neuraminidase abolished infection of HAE by human parainfluenza virus type 3, this treatment did not significantly affect infection by influenza viruses. All human viruses replicated efficiently in HAE, leading to accumulation of nascent virus released from the apical surface between 6 and 24 h postinfection with a low multiplicity of infection. Avian influenza A viruses also infected HAE, but spread was limited compared to that of human viruses. The nonciliated cell tropism of recent human H3N2 viruses reflects a preference for the sialic acid linkages displayed on these cell types and suggests a drift in the receptor binding phenotype of the H3 hemagglutinin protein as it evolves in humans away from its avian virus precursor.
Figures
Similar articles
-
Tropism and infectivity of influenza virus, including highly pathogenic avian H5N1 virus, in ferret tracheal differentiated primary epithelial cell cultures.J Virol. 2013 Mar;87(5):2597-607. doi: 10.1128/JVI.02885-12. Epub 2012 Dec 19. J Virol. 2013. PMID: 23255802 Free PMC article.
-
Tropism and Infectivity of a Seasonal A(H1N1) and a Highly Pathogenic Avian A(H5N1) Influenza Virus in Primary Differentiated Ferret Nasal Epithelial Cell Cultures.J Virol. 2019 May 1;93(10):e00080-19. doi: 10.1128/JVI.00080-19. Print 2019 May 15. J Virol. 2019. PMID: 30814288 Free PMC article.
-
Infection of ciliated cells by human parainfluenza virus type 3 in an in vitro model of human airway epithelium.J Virol. 2005 Jan;79(2):1113-24. doi: 10.1128/JVI.79.2.1113-1124.2005. J Virol. 2005. PMID: 15613339 Free PMC article.
-
Tissue and host tropism of influenza viruses: importance of quantitative analysis.Sci China C Life Sci. 2009 Dec;52(12):1101-10. doi: 10.1007/s11427-009-0161-x. Epub 2009 Dec 17. Sci China C Life Sci. 2009. PMID: 20016966 Review.
-
The hemagglutinin: a determinant of pathogenicity.Curr Top Microbiol Immunol. 2014;385:3-34. doi: 10.1007/82_2014_384. Curr Top Microbiol Immunol. 2014. PMID: 25031010 Review.
Cited by
-
Tropism and infectivity of influenza virus, including highly pathogenic avian H5N1 virus, in ferret tracheal differentiated primary epithelial cell cultures.J Virol. 2013 Mar;87(5):2597-607. doi: 10.1128/JVI.02885-12. Epub 2012 Dec 19. J Virol. 2013. PMID: 23255802 Free PMC article.
-
Influenza H5N1 virus infection of polarized human alveolar epithelial cells and lung microvascular endothelial cells.Respir Res. 2009 Oct 30;10(1):102. doi: 10.1186/1465-9921-10-102. Respir Res. 2009. PMID: 19874627 Free PMC article.
-
SARS-CoV-2 infection of airway cells causes intense viral and cell shedding, two spreading mechanisms affected by IL-13.Proc Natl Acad Sci U S A. 2022 Apr 19;119(16):e2119680119. doi: 10.1073/pnas.2119680119. Epub 2022 Mar 30. Proc Natl Acad Sci U S A. 2022. PMID: 35353667 Free PMC article.
-
Pathogenesis of avian influenza (H7) virus infection in mice and ferrets: enhanced virulence of Eurasian H7N7 viruses isolated from humans.J Virol. 2007 Oct;81(20):11139-47. doi: 10.1128/JVI.01235-07. Epub 2007 Aug 8. J Virol. 2007. PMID: 17686867 Free PMC article.
-
Human parainfluenza virus type 2 V protein inhibits interferon production and signaling and is required for replication in non-human primates.Virology. 2010 Feb 20;397(2):285-98. doi: 10.1016/j.virol.2009.11.018. Epub 2009 Dec 7. Virology. 2010. PMID: 19969320 Free PMC article.
References
-
- Abed, Y., A.-M. Bourgault, R. J. Fenton, P. J. Morley, D. Gower, I. J. Owens, M. Tisdale, and G. Boivin. 2002. Characterization of two influenza A (H3N2) clinical isolates with reduced susceptibility to neuraminidase inhibitors due to mutations in the hemagglutinin gene. J. Infect. Dis. 186:1074-1080. - PubMed
-
- Baum, L. G., and J. C. Paulson. 1990. Sialyloligosaccharides of the respiratory epithelium in the selection of human influenza virus receptor specificity. Acta Histochem. Suppl. 40:35-38. - PubMed
-
- Chotpitayasunondh, T., K. Ungchusak, W. Hanshaoworakul, S. Chunsuthiwat, P. Sawanpanyalert, R. Kijphati, S. Lochindarat, P. Srisan, P. Suwan, Y. Osotthanakorn, T. Anantasetagoon, S. Kanjanawasri, S. Tanupattarachai, J. Weerakul, R. Chaiwirattana, M. Maneerattanaporn, R. Poolsavathitikool, K. Chokephaibulkit, A. Apisarnthanarak, and S. F. Dowell. 2005. Human disease from influenza A (H5N1), Thailand, 2004. Emerg. Infect. Dis. 11:201-209. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

