Rinderpest virus blocks type I and type II interferon action: role of structural and nonstructural proteins
- PMID: 16840335
- PMCID: PMC1563703
- DOI: 10.1128/JVI.02720-05
Rinderpest virus blocks type I and type II interferon action: role of structural and nonstructural proteins
Abstract
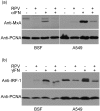
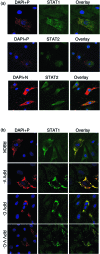
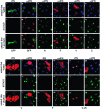
Rinderpest virus (RPV) is a paramyxovirus closely related to the human pathogen Measles virus. It causes severe disease in cattle, buffalo, and some wild animals; although it can infect humans, it does not cause disease. Here, we demonstrate that RPV blocks the action of both type I (alpha) and type II (gamma) interferons (IFNs) by blocking the phosphorylation and nuclear translocation of STAT1 and STAT2 and that this block is not related to species specificity. In addition, both wild-type virulent and vaccine strains of the virus blocked IFN action. Unlike the case with some other paramyxoviruses, neither STAT1 nor STAT2 is degraded upon virus infection. STAT1 is bound by both the viral structural protein P, and thereby recruited to concentrations of viral protein in the cell, and the nonstructural protein V. Although both P and V proteins bind to STAT1 and can block IFN action when expressed in transfected cells, the IFN antagonist activity of the P protein is weaker than that of the V protein. The viral C protein also seems to weakly block IFN-induced activation of STAT1 in transfection experiments. However, studies with knockout viruses showed that the viral V protein appears to be the dominant inhibitor of IFN signaling in the context of virus infection, since prevention of viral V expression restored the IFN sensitivity of infected cells. Although a change in the distribution pattern of STAT2 was observed in virus-infected cells, STAT2 was not bound by any viral protein.
Figures


Similar articles
-
Morbillivirus v proteins exhibit multiple mechanisms to block type 1 and type 2 interferon signalling pathways.PLoS One. 2013;8(2):e57063. doi: 10.1371/journal.pone.0057063. Epub 2013 Feb 19. PLoS One. 2013. PMID: 23431397 Free PMC article.
-
Heartland virus antagonizes type I and III interferon antiviral signaling by inhibiting phosphorylation and nuclear translocation of STAT2 and STAT1.J Biol Chem. 2019 Jun 14;294(24):9503-9517. doi: 10.1074/jbc.RA118.006563. Epub 2019 Apr 30. J Biol Chem. 2019. PMID: 31040183 Free PMC article.
-
Interferon-gamma inhibits interferon-alpha signalling in hepatic cells: evidence for the involvement of STAT1 induction and hyperexpression of STAT1 in chronic hepatitis C.Biochem J. 2004 Apr 1;379(Pt 1):199-208. doi: 10.1042/BJ20031495. Biochem J. 2004. PMID: 14690454 Free PMC article.
-
Unphosphorylated ISGF3 drives constitutive expression of interferon-stimulated genes to protect against viral infections.Sci Signal. 2017 Apr 25;10(476):eaah4248. doi: 10.1126/scisignal.aah4248. Sci Signal. 2017. PMID: 28442624
-
The rinderpest virus non-structural C protein blocks the induction of type 1 interferon.Virology. 2009 Mar 1;385(1):134-42. doi: 10.1016/j.virol.2008.11.022. Epub 2008 Dec 23. Virology. 2009. PMID: 19108859
Cited by
-
The Nucleoprotein and Phosphoprotein of Peste des Petits Ruminants Virus Inhibit Interferons Signaling by Blocking the JAK-STAT Pathway.Viruses. 2019 Jul 8;11(7):629. doi: 10.3390/v11070629. Viruses. 2019. PMID: 31288481 Free PMC article.
-
Peste des petits ruminants virus infection of small ruminants: a comprehensive review.Viruses. 2014 Jun 6;6(6):2287-327. doi: 10.3390/v6062287. Viruses. 2014. PMID: 24915458 Free PMC article. Review.
-
C Proteins: Controllers of Orderly Paramyxovirus Replication and of the Innate Immune Response.Viruses. 2022 Jan 12;14(1):137. doi: 10.3390/v14010137. Viruses. 2022. PMID: 35062341 Free PMC article. Review.
-
Evasion of Host Antiviral Innate Immunity by Paramyxovirus Accessory Proteins.Front Microbiol. 2022 Jan 31;12:790191. doi: 10.3389/fmicb.2021.790191. eCollection 2021. Front Microbiol. 2022. PMID: 35173691 Free PMC article. Review.
-
Antibodies to the core proteins of Nairobi sheep disease virus/Ganjam virus reveal details of the distribution of the proteins in infected cells and tissues.PLoS One. 2015 Apr 23;10(4):e0124966. doi: 10.1371/journal.pone.0124966. eCollection 2015. PLoS One. 2015. PMID: 25905707 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

