MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia
- PMID: 16834459
- PMCID: PMC1502153
- DOI: 10.1371/journal.pmed.0030270
MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia
Abstract
Background: The JAK2V617F allele has recently been identified in patients with polycythemia vera (PV), essential thrombocytosis (ET), and myelofibrosis with myeloid metaplasia (MF). Subsequent analysis has shown that constitutive activation of the JAK-STAT signal transduction pathway is an important pathogenetic event in these patients, and that enzymatic inhibition of JAK2V617F may be of therapeutic benefit in this context. However, a significant proportion of patients with ET or MF are JAK2V617F-negative. We hypothesized that activation of the JAK-STAT pathway might also occur as a consequence of activating mutations in certain hematopoietic-specific cytokine receptors, including the erythropoietin receptor (EPOR), the thrombopoietin receptor (MPL), or the granulocyte-colony stimulating factor receptor (GCSFR).
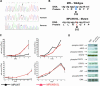
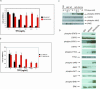
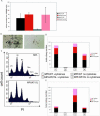
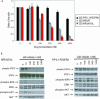
Methods and findings: DNA sequence analysis of the exons encoding the transmembrane and juxtamembrane domains of EPOR, MPL, and GCSFR, and comparison with germline DNA derived from buccal swabs, identified a somatic activating mutation in the transmembrane domain of MPL (W515L) in 9% (4/45) of JAKV617F-negative MF. Expression of MPLW515L in 32D, UT7, or Ba/F3 cells conferred cytokine-independent growth and thrombopoietin hypersensitivity, and resulted in constitutive phosphorylation of JAK2, STAT3, STAT5, AKT, and ERK. Furthermore, a small molecule JAK kinase inhibitor inhibited MPLW515L-mediated proliferation and JAK-STAT signaling in vitro. In a murine bone marrow transplant assay, expression of MPLW515L, but not wild-type MPL, resulted in a fully penetrant myeloproliferative disorder characterized by marked thrombocytosis (Plt count 1.9-4.0 x 10(12)/L), marked splenomegaly due to extramedullary hematopoiesis, and increased reticulin fibrosis.
Conclusions: Activation of JAK-STAT signaling via MPLW515L is an important pathogenetic event in patients with JAK2V617F-negative MF. The bone marrow transplant model of MPLW515L-mediated myeloproliferative disorders (MPD) exhibits certain features of human MF, including extramedullary hematopoiesis, splenomegaly, and megakaryocytic proliferation. Further analysis of positive and negative regulators of the JAK-STAT pathway is warranted in JAK2V617F-negative MPD.
Conflict of interest statement
Figures



Similar articles
-
Role of JAK-STAT signaling in the pathogenesis of myeloproliferative disorders.Hematology Am Soc Hematol Educ Program. 2006:233-9, 510. doi: 10.1182/asheducation-2006.1.233. Hematology Am Soc Hematol Educ Program. 2006. PMID: 17124066 Review.
-
Efficacy of the JAK2 inhibitor INCB16562 in a murine model of MPLW515L-induced thrombocytosis and myelofibrosis.Blood. 2010 Apr 8;115(14):2919-27. doi: 10.1182/blood-2009-04-218842. Epub 2010 Feb 12. Blood. 2010. PMID: 20154217 Free PMC article.
-
TG101209, a small molecule JAK2-selective kinase inhibitor potently inhibits myeloproliferative disorder-associated JAK2V617F and MPLW515L/K mutations.Leukemia. 2007 Aug;21(8):1658-68. doi: 10.1038/sj.leu.2404750. Epub 2007 May 31. Leukemia. 2007. PMID: 17541402
-
The small molecule inhibitor G6 significantly reduces bone marrow fibrosis and the mutant burden in a mouse model of Jak2-mediated myelofibrosis.Am J Pathol. 2012 Sep;181(3):858-65. doi: 10.1016/j.ajpath.2012.05.033. Epub 2012 Jul 13. Am J Pathol. 2012. PMID: 22796437 Free PMC article.
-
JAK2 and MPL mutations in myeloproliferative neoplasms.Acta Haematol. 2008;119(4):218-25. doi: 10.1159/000140634. Epub 2008 Jun 20. Acta Haematol. 2008. PMID: 18566540 Review.
Cited by
-
Molecular insights into regulation of JAK2 in myeloproliferative neoplasms.Blood. 2015 May 28;125(22):3388-92. doi: 10.1182/blood-2015-01-621110. Epub 2015 Mar 30. Blood. 2015. PMID: 25824690 Free PMC article. Review.
-
Chromosome 9p trisomy increases stem cells clonogenic potential and fosters T-cell exhaustion in JAK2-mutant myeloproliferative neoplasms.Leukemia. 2024 Oct;38(10):2171-2182. doi: 10.1038/s41375-024-02373-w. Epub 2024 Aug 23. Leukemia. 2024. PMID: 39179669 Free PMC article.
-
Cancer Immune Therapy for Philadelphia Chromosome-Negative Chronic Myeloproliferative Neoplasms.Cancers (Basel). 2020 Jul 2;12(7):1763. doi: 10.3390/cancers12071763. Cancers (Basel). 2020. PMID: 32630667 Free PMC article. Review.
-
Molecular analyses of 15,542 patients with suspected BCR-ABL1-negative myeloproliferative disorders allow to develop a stepwise diagnostic workflow.Haematologica. 2012 Oct;97(10):1582-5. doi: 10.3324/haematol.2012.064683. Epub 2012 Apr 17. Haematologica. 2012. PMID: 22511494 Free PMC article.
-
Molecular Genetic Profile of Myelofibrosis: Implications in the Diagnosis, Prognosis, and Treatment Advancements.Cancers (Basel). 2024 Jan 25;16(3):514. doi: 10.3390/cancers16030514. Cancers (Basel). 2024. PMID: 38339265 Free PMC article. Review.
References
-
- Dameshek W. Some speculations on the myeloproliferative syndromes. Blood. 1951;6:372–375. - PubMed
-
- Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–397. - PubMed
-
- Jones AV, Kreil S, Zoi K, Waghorn K, Curtis C, et al. Widespread occurrence of the JAK2 V617F mutation in chronic myeloproliferative disorders. Blood. 2005;106:2162–2168. - PubMed
-
- James C, Ugo V, Le Couedic JP, StaERK J, Delhommeau F, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–1148. - PubMed
-
- Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–1061. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

