CD4+ T-cell memory: generation and multi-faceted roles for CD4+ T cells in protective immunity to influenza
- PMID: 16824113
- PMCID: PMC2266984
- DOI: 10.1111/j.0105-2896.2006.00388.x
CD4+ T-cell memory: generation and multi-faceted roles for CD4+ T cells in protective immunity to influenza
Erratum in
- Immunol Rev. 2006 Oct;213:256
Abstract
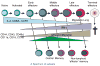
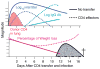
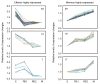
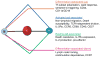
We have outlined the carefully orchestrated process of CD4+ T-cell differentiation from naïve to effector and from effector to memory cells with a focus on how these processes can be studied in vivo in responses to pathogen infection. We emphasize that the regulatory factors that determine the quality and quantity of the effector and memory cells generated include (i) the antigen dose during the initial T-cell interaction with antigen-presenting cells; (ii) the dose and duration of repeated interactions; and (iii) the milieu of inflammatory and growth cytokines that responding CD4+ T cells encounter. We suggest that heterogeneity in these regulatory factors leads to the generation of a spectrum of effectors with different functional attributes. Furthermore, we suggest that it is the presence of effectors at different stages along a pathway of progressive linear differentiation that leads to a related spectrum of memory cells. Our studies particularly highlight the multifaceted roles of CD4+ effector and memory T cells in protective responses to influenza infection and support the concept that efficient priming of CD4+ T cells that react to shared influenza proteins could contribute greatly to vaccine strategies for influenza.
Figures






Similar articles
-
Short-Lived Antigen Recognition but Not Viral Infection at a Defined Checkpoint Programs Effector CD4 T Cells To Become Protective Memory.J Immunol. 2016 Nov 15;197(10):3936-3949. doi: 10.4049/jimmunol.1600838. Epub 2016 Oct 17. J Immunol. 2016. PMID: 27798159 Free PMC article.
-
Fucosyltransferase Induction during Influenza Virus Infection Is Required for the Generation of Functional Memory CD4+ T Cells.J Immunol. 2018 Apr 15;200(8):2690-2702. doi: 10.4049/jimmunol.1701251. Epub 2018 Feb 28. J Immunol. 2018. PMID: 29491007 Free PMC article.
-
Unexpected prolonged presentation of influenza antigens promotes CD4 T cell memory generation.J Exp Med. 2005 Sep 5;202(5):697-706. doi: 10.1084/jem.20050227. J Exp Med. 2005. PMID: 16147980 Free PMC article.
-
Influencing the fates of CD4 T cells on the path to memory: lessons from influenza.Immunol Cell Biol. 2008 May-Jun;86(4):343-52. doi: 10.1038/icb.2008.13. Epub 2008 Mar 25. Immunol Cell Biol. 2008. PMID: 18362946 Review.
-
CD4 T cells in protection from influenza virus: Viral antigen specificity and functional potential.Immunol Rev. 2018 Jul;284(1):91-105. doi: 10.1111/imr.12662. Immunol Rev. 2018. PMID: 29944766 Free PMC article. Review.
Cited by
-
A Recombinant Mosaic HAs Influenza Vaccine Elicits Broad-Spectrum Immune Response and Protection of Influenza a Viruses.Vaccines (Basel). 2024 Sep 2;12(9):1008. doi: 10.3390/vaccines12091008. Vaccines (Basel). 2024. PMID: 39340038 Free PMC article.
-
The dynamics and longevity of circulating CD4+ memory T cells depend on cell age and not the chronological age of the host.PLoS Biol. 2024 Aug 13;22(8):e3002380. doi: 10.1371/journal.pbio.3002380. eCollection 2024 Aug. PLoS Biol. 2024. PMID: 39137219 Free PMC article.
-
The dynamics and longevity of circulating CD4+ memory T cells depend on cell age and not the chronological age of the host.bioRxiv [Preprint]. 2024 Jun 25:2023.10.16.562650. doi: 10.1101/2023.10.16.562650. bioRxiv. 2024. Update in: PLoS Biol. 2024 Aug 13;22(8):e3002380. doi: 10.1371/journal.pbio.3002380. PMID: 38948729 Free PMC article. Updated. Preprint.
-
Diet-induced obesity affects influenza disease severity and transmission dynamics in ferrets.Sci Adv. 2024 May 10;10(19):eadk9137. doi: 10.1126/sciadv.adk9137. Epub 2024 May 10. Sci Adv. 2024. PMID: 38728395 Free PMC article.
-
SARS-CoV-2 infection risk is higher in vaccinated patients with inflammatory autoimmune diseases or liver transplantation treated with mycophenolate due to an impaired antiviral immune response: results of the extended follow up of the RIVALSA prospective cohort.Front Immunol. 2023 Jul 20;14:1185278. doi: 10.3389/fimmu.2023.1185278. eCollection 2023. Front Immunol. 2023. PMID: 37545528 Free PMC article.
References
-
- Swain SL. Lymphocyte effector functions – lymphocyte heterogeneity – is it limitless? Curr Opin Immunol. 2003;15:332–335.
-
- Finkelman FD, et al. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–333. - PubMed
-
- McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific memory B cell development. Annu Rev Immunol. 2005;23:487–513. - PubMed
-
- Garside P, Ingulli E, Merica RR, Johnson JG, Noelle RJ, Jenkins MK. Visualization of specific B and T lymphocyte interactions in the lymph node. Science. 1998;281:96–99. - PubMed
-
- Bevan MJ. Helping the CD8 (+) T-cell response. Nat Rev Immunol. 2004;4:595–602. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

