Glucose transport and sensing in the maintenance of glucose homeostasis and metabolic harmony
- PMID: 16823474
- PMCID: PMC1483149
- DOI: 10.1172/JCI29027
Glucose transport and sensing in the maintenance of glucose homeostasis and metabolic harmony
Abstract
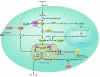
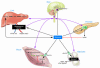
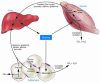
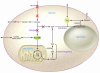
Recent data underscore the importance of intertissue communication in the maintenance of normal glucose homeostasis. Important signals are conveyed by hormones, cytokines, and fuel substrates and are sensed through a variety of cellular mechanisms. The ability of tissues to sense and adapt to changes in metabolic status and fuel availability is altered in insulin-resistant states including type 2 diabetes. Here we review the roles of glucose and its metabolites as signaling molecules and the diverse physiologic mechanisms for glucose sensing.
Figures
Similar articles
-
Sensing the fuels: glucose and lipid signaling in the CNS controlling energy homeostasis.Cell Mol Life Sci. 2010 Oct;67(19):3255-73. doi: 10.1007/s00018-010-0414-7. Epub 2010 Jun 12. Cell Mol Life Sci. 2010. PMID: 20549539 Free PMC article. Review.
-
Role of insulin, adipocyte hormones, and nutrient-sensing pathways in regulating fuel metabolism and energy homeostasis: a nutritional perspective of diabetes, obesity, and cancer.Sci STKE. 2006 Aug 1;2006(346):re7. doi: 10.1126/stke.3462006re7. Sci STKE. 2006. PMID: 16885148 Review.
-
An adipocentric view of signaling and intracellular trafficking.Diabetes Metab Res Rev. 2002 Sep-Oct;18(5):345-56. doi: 10.1002/dmrr.321. Diabetes Metab Res Rev. 2002. PMID: 12397577 Review.
-
Amino Acid Sensing in Metabolic Homeostasis and Health.Endocr Rev. 2021 Jan 28;42(1):56-76. doi: 10.1210/endrev/bnaa026. Endocr Rev. 2021. PMID: 33053153 Review.
-
CNS-targets in control of energy and glucose homeostasis.Curr Opin Pharmacol. 2009 Dec;9(6):794-804. doi: 10.1016/j.coph.2009.10.006. Epub 2009 Oct 31. Curr Opin Pharmacol. 2009. PMID: 19884043 Review.
Cited by
-
Regulation of adipocyte differentiation and metabolism by lansoprazole.Life Sci. 2019 Dec 15;239:116897. doi: 10.1016/j.lfs.2019.116897. Epub 2019 Oct 20. Life Sci. 2019. PMID: 31644894 Free PMC article.
-
Heat-Killed Lactiplantibacillus plantarum LRCC5314 Mitigates the Effects of Stress-Related Type 2 Diabetes in Mice via Gut Microbiome Modulation.J Microbiol Biotechnol. 2022 Mar 28;32(3):324-332. doi: 10.4014/jmb.2111.11008. J Microbiol Biotechnol. 2022. PMID: 34949748 Free PMC article.
-
Nonalcoholic fatty liver disease: an overview of current insights in pathogenesis, diagnosis and treatment.World J Gastroenterol. 2008 Apr 28;14(16):2474-86. doi: 10.3748/wjg.14.2474. World J Gastroenterol. 2008. PMID: 18442193 Free PMC article. Review.
-
A closed-loop multi-level model of glucose homeostasis.PLoS One. 2018 Feb 8;13(2):e0190627. doi: 10.1371/journal.pone.0190627. eCollection 2018. PLoS One. 2018. PMID: 29420588 Free PMC article.
-
Induction of the ChREBPβ Isoform Is Essential for Glucose-Stimulated β-Cell Proliferation.Diabetes. 2015 Dec;64(12):4158-70. doi: 10.2337/db15-0239. Epub 2015 Sep 17. Diabetes. 2015. PMID: 26384380 Free PMC article.
References
-
- Mokdad A.H., et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. Jama. 2003;289:76–79. - PubMed
-
- McGarry J.D. What if Minkowski had been ageusic? An alternative angle on diabetes. Science. 1992;258:766–770. - PubMed
-
- Shepherd P.R., Kahn B.B. Glucose transporters and insulin action — implications for insulin resistance and diabetes mellitus. N. Engl. J. Med. 1999;341:248–257. - PubMed
-
- DeFronzo R. Pathogenesis of type 2 diabetes: metabolic and molecular implications for identifying diabetes genes. Diabetes Reviews. 1997;5:177–269.
-
- Storlien L., Oakes N.D., Kelley D.E. Metabolic flexibility. Proc. Nutr. Soc. 2004;63:363–368. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

