Dynamic and transient remodeling of the macrophage IL-10 promoter during transcription
- PMID: 16818788
- PMCID: PMC2643023
- DOI: 10.4049/jimmunol.177.2.1282
Dynamic and transient remodeling of the macrophage IL-10 promoter during transcription
Abstract
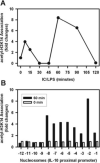
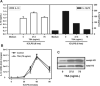
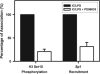
To gain insight into the molecular mechanism(s) whereby macrophages produce large amounts of IL-10, we analyzed IL-10 gene expression and temporally correlated it with modifications to chromatin associated with the IL-10 promoter. In resting cells, which make essentially no cytokines, the IL-10 promoter is associated with histones containing little or no detectable modifications. Macrophages stimulated in the presence of immune complexes begin to produce high levels of IL-10 pre-mRNA transcripts within minutes of stimulation. Coincident with this transcription was a rapid and dynamic phosphorylation of histone H3 at specific sites in the IL-10 promoter. Histone phosphorylation was closely followed by the binding of transcription factors to the IL-10 promoter. Blocking the activation of ERK prevented histone phosphorylation and transcription factor binding to the IL-10 promoter. In contrast to histone phosphorylation, the peak of histone acetylation at this promoter did not occur until after transcription had peaked. Inhibition of histone deactylase did not alter IL-10 gene expression, suggesting that phosphorylation but not acetylation was the proximal event responsible for IL-10 transcription. Our findings reveal a rapid and well-orchestrated series of events in which ERK activation causes a rapid and transient phosphorylation of histone H3 at specific regions of the IL-10 promoter, resulting in a transient exposure of the IL-10 promoter to the transcription factors that bind there. This exposure is essential for the efficient induction of IL-10 gene expression in macrophages. To our knowledge, this represents a unique way in which the expression of a cytokine gene is regulated in macrophages.
Figures







Similar articles
-
ERK activation following macrophage FcgammaR ligation leads to chromatin modifications at the IL-10 locus.J Immunol. 2005 Jul 1;175(1):469-77. doi: 10.4049/jimmunol.175.1.469. J Immunol. 2005. PMID: 15972681
-
Intracellular bacteria differentially regulated endothelial cytokine release by MAPK-dependent histone modification.J Immunol. 2005 Sep 1;175(5):2843-50. doi: 10.4049/jimmunol.175.5.2843. J Immunol. 2005. PMID: 16116170
-
Functional cooperation of simian virus 40 promoter factor 1 and CCAAT/enhancer-binding protein beta and delta in lipopolysaccharide-induced gene activation of IL-10 in mouse macrophages.J Immunol. 2003 Jul 15;171(2):821-8. doi: 10.4049/jimmunol.171.2.821. J Immunol. 2003. PMID: 12847250
-
Epigenetic and transcriptional mechanisms for the regulation of IL-10.Semin Immunol. 2019 Aug;44:101324. doi: 10.1016/j.smim.2019.101324. Epub 2019 Oct 30. Semin Immunol. 2019. PMID: 31676122 Free PMC article. Review.
-
The regulation of IL-10 production by immune cells.Nat Rev Immunol. 2010 Mar;10(3):170-81. doi: 10.1038/nri2711. Epub 2010 Feb 15. Nat Rev Immunol. 2010. PMID: 20154735 Review.
Cited by
-
Computational System Level Approaches for Discerning Reciprocal Regulation of IL10 and IL12 in Leishmaniasis.Front Genet. 2022 Jan 19;12:784664. doi: 10.3389/fgene.2021.784664. eCollection 2021. Front Genet. 2022. PMID: 35126456 Free PMC article.
-
Searching for the Transcriptomic Signature of Immune Tolerance Induction-Biomarkers of Safety and Functionality for Tolerogenic Dendritic Cells and Regulatory Macrophages.Front Immunol. 2018 Sep 19;9:2062. doi: 10.3389/fimmu.2018.02062. eCollection 2018. Front Immunol. 2018. PMID: 30298066 Free PMC article. Review.
-
A helminth immunomodulator exploits host signaling events to regulate cytokine production in macrophages.PLoS Pathog. 2011 Jan 6;7(1):e1001248. doi: 10.1371/journal.ppat.1001248. PLoS Pathog. 2011. PMID: 21253577 Free PMC article.
-
Toxoplasma gondii prevents chromatin remodeling initiated by TLR-triggered macrophage activation.J Immunol. 2009 Jan 1;182(1):489-97. doi: 10.4049/jimmunol.182.1.489. J Immunol. 2009. PMID: 19109180 Free PMC article.
-
Chronic Lymphocytic Leukemia-Derived IL-10 Suppresses Antitumor Immunity.J Immunol. 2018 Jun 15;200(12):4180-4189. doi: 10.4049/jimmunol.1800241. Epub 2018 Apr 30. J Immunol. 2018. PMID: 29712773 Free PMC article.
References
-
- van der Poll T, van Deventer SJ. Cytokines and anticytokines in the pathogenesis of sepsis. Infect. Dis. Clin. North Am. 1999;13:413–426. - PubMed
-
- Waage A, Aasen AO. Different role of cytokine mediators in septic shock related to meningococcal disease and surgery/polytrauma. Immunol. Rev. 1992;127:221–230. - PubMed
-
- Kawai T, Akira S. Pathogen recognition with Toll-like receptors. Curr. Opin. Immunol. 2005;17:338–344. - PubMed
-
- Zdanov A. Structural features of the interleukin-10 family of cytokines. Curr. Pharm. Des. 2004;10:3873–3884. - PubMed
-
- Donnelly RP, Sheikh F, Kotenko SV, Dickensheets H. The expanded family of class II cytokines that share the IL-10 receptor-2 (IL-10R2) chain. J. Leukocyte Biol. 2004;76:314–321. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

