Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis
- PMID: 16785311
- PMCID: PMC2118357
- DOI: 10.1084/jem.20052362
Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis
Abstract
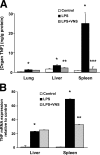
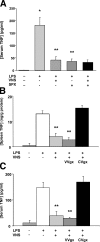
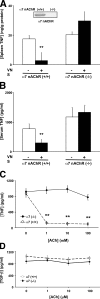
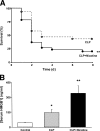
The innate immune system protects against infection and tissue injury through the specialized organs of the reticuloendothelial system, including the lungs, liver, and spleen. The central nervous system regulates innate immune responses via the vagus nerve, a mechanism termed the cholinergic antiinflammatory pathway. Vagus nerve stimulation inhibits proinflammatory cytokine production by signaling through the alpha7 nicotinic acetylcholine receptor subunit. Previously, the functional relationship between the cholinergic antiinflammatory pathway and the reticuloendothelial system was unknown. Here we show that vagus nerve stimulation fails to inhibit tumor necrosis factor (TNF) production in splenectomized animals during lethal endotoxemia. Selective lesioning of the common celiac nerve abolishes TNF suppression by vagus nerve stimulation, suggesting that the cholinergic pathway is functionally hard wired to the spleen via this branch of the vagus nerve. Administration of nicotine, an alpha7 agonist that mimics vagus nerve stimulation, increases proinflammatory cytokine production and lethality from polymicrobial sepsis in splenectomized mice, indicating that the spleen is critical to the protective response of the cholinergic pathway. These results reveal a specific, physiological connection between the nervous and innate immune systems that may be exploited through either electrical vagus nerve stimulation or administration of alpha7 agonists to inhibit proinflammatory cytokine production during infection and tissue injury.
Figures
Similar articles
-
Transcutaneous vagus nerve stimulation reduces serum high mobility group box 1 levels and improves survival in murine sepsis.Crit Care Med. 2007 Dec;35(12):2762-8. doi: 10.1097/01.CCM.0000288102.15975.BA. Crit Care Med. 2007. PMID: 17901837
-
Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia.Proc Natl Acad Sci U S A. 2008 Aug 5;105(31):11008-13. doi: 10.1073/pnas.0803237105. Epub 2008 Jul 31. Proc Natl Acad Sci U S A. 2008. PMID: 18669662 Free PMC article.
-
Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation.Nature. 2003 Jan 23;421(6921):384-8. doi: 10.1038/nature01339. Epub 2002 Dec 22. Nature. 2003. PMID: 12508119
-
The vagus nerve and the inflammatory reflex: wandering on a new treatment paradigm for systemic inflammation and sepsis.Surg Infect (Larchmt). 2012 Aug;13(4):187-93. doi: 10.1089/sur.2012.126. Epub 2012 Aug 22. Surg Infect (Larchmt). 2012. PMID: 22913335 Review.
-
Role of cholinergic anti-inflammatory pathway in regulating host response and its interventional strategy for inflammatory diseases.Chin J Traumatol. 2009 Dec;12(6):355-64. Chin J Traumatol. 2009. PMID: 19930906 Review.
Cited by
-
Neuronal and nonneuronal cholinergic structures in the mouse gastrointestinal tract and spleen.J Comp Neurol. 2013 Nov;521(16):3741-67. doi: 10.1002/cne.23376. J Comp Neurol. 2013. PMID: 23749724 Free PMC article.
-
Rib detection using pitch-catch ultrasound and classification algorithms for a novel ultrasound therapy device.Bioelectron Med. 2023 Nov 15;9(1):25. doi: 10.1186/s42234-023-00127-0. Bioelectron Med. 2023. PMID: 37964380 Free PMC article.
-
Evaluating the effects of protective ventilation on organ-specific cytokine production in porcine experimental postoperative sepsis.BMC Pulm Med. 2015 May 10;15:60. doi: 10.1186/s12890-015-0052-9. BMC Pulm Med. 2015. PMID: 25958003 Free PMC article.
-
The role of the vagus nerve: modulation of the inflammatory reaction in murine polymicrobial sepsis.Mediators Inflamm. 2012;2012:467620. doi: 10.1155/2012/467620. Epub 2012 Mar 21. Mediators Inflamm. 2012. PMID: 22547905 Free PMC article.
-
Loss of intestinal sympathetic innervation elicits an innate immune driven colitis.Mol Med. 2019 Jan 7;25(1):1. doi: 10.1186/s10020-018-0068-8. Mol Med. 2019. PMID: 30616543 Free PMC article.
References
-
- Angus, D.C., W.T. Linde-Zwirble, J. Lidicker, G. Clermont, J. Carcillo, and M.R. Pinsky. 2001. Epidemiology of severe sepsis in the United States: analyses of incidence, outcome, and associated costs of care. Crit. Care Med. 29:1303–1310. - PubMed
-
- Nathan, C. 2002. Points of control in inflammation. Nature. 420:846–852. - PubMed
-
- Ulloa, L., and K.J. Tracey. 2005. The “cytokine profile”: a code for sepsis. Trends Mol. Med. 11:56–63. - PubMed
-
- Tracey, K.J. 2005. Fatal Sequence: The Killer Within. Dana Press, Washington, DC. 225 pp.
-
- Tracey, K.J. 2002. The inflammatory reflex. Nature. 420:853–859. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

