TRPC1 protects human SH-SY5Y cells against salsolinol-induced cytotoxicity by inhibiting apoptosis
- PMID: 16765919
- PMCID: PMC2845452
- DOI: 10.1016/j.brainres.2006.04.104
TRPC1 protects human SH-SY5Y cells against salsolinol-induced cytotoxicity by inhibiting apoptosis
Abstract
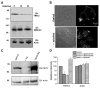
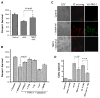
Salsolinol, an endogenous neurotoxin, may be involved in the pathogenesis of Parkinson's disease. In this study, we sought to determine whether salsolinol-induced cytotoxicity in SH-SY5Y human neuroblastoma cells, a cloned cell line which expresses dopaminergic activity, could be prevented by overexpressing a Ca(2+) channel, transient receptor potential (TRPC1) protein. Exposure of SH-SY5Y cells to 500 microM salsolinol for 12 h resulted in a significant decrease in thapsigargin or carbachol-mediated Ca(2+) influx. Consistent with these results, SH-SY5Y cells treated with salsolinol showed approximately 60% reduction in TRPC1 protein levels. Confocal microscopy also showed that SH-SY5Y cells treated with salsolinol had a significant decrease in the plasma membrane staining of the TRPC1 protein. Interestingly, overexpression of TRPC1 increases TRPC1 protein levels and also protected SH-SY5Y neuroblastoma cells against salsolinol-mediated cytotoxicity as determined by 3,[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay. The protective effect of TRPC1 was blocked by the addition of TRPC1 blockers lanthanum, or 2APB. Activation of TRPC1 protein by either thapsigargin or carbachol further protected SH-SY5Y cells from salsolinol treatments. Staining of SH-SY5Y cells with an apoptotic marker (YO-PRO-1) showed that TRPC1 protein protects against apoptosis. Furthermore, TRPC1 overexpression also inhibited cytochrome c release and decreased BAX protein levels required for apoptosis. Taken together, these findings suggest that the reduction in cell surface TRPC1 protein expression in response to salsolinol may be a contributory factor in cellular toxicity of the dopaminergic neurons. Furthermore, overexpression of TRPC1 could inhibit apoptotic complex thereby increasing neuronal cell survivability in Parkinson's disease.
Figures
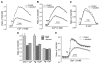
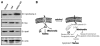
Similar articles
-
TRPC1 protects dopaminergic SH-SY5Y cells from MPP+, salsolinol, and N-methyl-(R)-salsolinol-induced cytotoxicity.Acta Biochim Biophys Sin (Shanghai). 2014 Jan;46(1):22-30. doi: 10.1093/abbs/gmt127. Epub 2013 Nov 18. Acta Biochim Biophys Sin (Shanghai). 2014. PMID: 24252728
-
TRPC1-mediated inhibition of 1-methyl-4-phenylpyridinium ion neurotoxicity in human SH-SY5Y neuroblastoma cells.J Biol Chem. 2005 Jan 21;280(3):2132-40. doi: 10.1074/jbc.M407384200. Epub 2004 Nov 12. J Biol Chem. 2005. PMID: 15542611 Free PMC article.
-
Low level of glutathione can intensify the toxic effect of salsolinol in SH-SY5Y neuroblastoma cell line.Neurotoxicology. 2012 Jun;33(3):424-8. doi: 10.1016/j.neuro.2012.04.007. Epub 2012 Apr 13. Neurotoxicology. 2012. PMID: 22525935
-
Apoptosis induced by an endogenous neurotoxin, N-methyl(R)salsolinol, in dopamine neurons.Toxicology. 2000 Nov 16;153(1-3):123-41. doi: 10.1016/s0300-483x(00)00309-7. Toxicology. 2000. PMID: 11090952 Review.
-
N-methyl-(R)salsolinol as a dopaminergic neurotoxin: from an animal model to an early marker of Parkinson's disease.J Neural Transm Suppl. 1997;50:89-105. doi: 10.1007/978-3-7091-6842-4_10. J Neural Transm Suppl. 1997. PMID: 9120428 Review.
Cited by
-
HIV-1 Tat-Mediated Calcium Dysregulation and Neuronal Dysfunction in Vulnerable Brain Regions.Curr Drug Targets. 2016;17(1):4-14. doi: 10.2174/1389450116666150531162212. Curr Drug Targets. 2016. PMID: 26028040 Free PMC article. Review.
-
Do calcium channel blockers rescue dying photoreceptors in the Pde6b ( rd1 ) mouse?Adv Exp Med Biol. 2010;664:491-9. doi: 10.1007/978-1-4419-1399-9_56. Adv Exp Med Biol. 2010. PMID: 20238051 Free PMC article. Review.
-
Physiology and pathophysiology of canonical transient receptor potential channels.FASEB J. 2009 Feb;23(2):297-328. doi: 10.1096/fj.08-119495. Epub 2008 Oct 21. FASEB J. 2009. PMID: 18940894 Free PMC article. Review.
-
Inhibition of L-Type Ca2+ Channels by TRPC1-STIM1 Complex Is Essential for the Protection of Dopaminergic Neurons.J Neurosci. 2017 Mar 22;37(12):3364-3377. doi: 10.1523/JNEUROSCI.3010-16.2017. Epub 2017 Mar 3. J Neurosci. 2017. PMID: 28258168 Free PMC article.
-
TRPC1 expression and function inhibit ER stress and cell death in salivary gland cells.FASEB Bioadv. 2019 Jan;1(1):40-50. doi: 10.1096/fba.1021. Epub 2018 Oct 8. FASEB Bioadv. 2019. PMID: 31111119 Free PMC article.
References
-
- Anglade P, Vyas S, Javoy-Agid F, Herrero MT, Michel PP, Marquez J, Mouatt-Prigent A, Ruberg M, Hirsch EC, Agid Y. Apoptosis and autophagy in nigral neurons of patients with Parkinson's disease. Histol Histopathol. 1997;12:25–31. - PubMed
-
- Baffy G, Miyashita T, Williamson JR, Reed JC. Apoptosis induced by withdrawal of interleukin-3 (IL-3) from an IL-3-dependent hematopoietic cell line is associated with repartitioning of intracellular calcium and is blocked by enforced Bcl-2 oncoprotein production. J Biol Chem. 1993;268:6511–6519. - PubMed
-
- Bellomo G, Perotti M, Taddei F, Mirabelli F, Finardi G, Nicotera P, Orrenius S. Tumor necrosis factor alpha induces apoptosis in mammary adenocarcinoma cells by an increase in intranuclear free Ca2+ concentration and DNA fragmentation. Cancer Res. 1992;52:1342–1346. - PubMed
-
- Bembenek ME, Abell CW, Chrisey LA, Rozwadowska MD, Gessner AW, Brossi A. Inhibition of monoamine oxidase-A and oxidase-B by simple isoquinoline alkaloids-racemic and optically active 1,2,3,4-tetrahydroisoquinoline, 3,4-dihydroisoquinoline, and fully aromatic isoquinoline. J Med Chem. 1983;33:147–152. - PubMed
-
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev, Mol Cell Biol. 2000;1:11–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

