A new Drosophila gene wh (wuho) with WD40 repeats is essential for spermatogenesis and has maximal expression in hub cells
- PMID: 16762337
- PMCID: PMC2055424
- DOI: 10.1016/j.ydbio.2006.04.459
A new Drosophila gene wh (wuho) with WD40 repeats is essential for spermatogenesis and has maximal expression in hub cells
Abstract
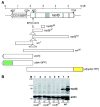
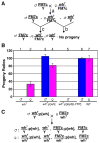
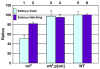
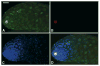
Through mutagenesis by P-element transposition, we identified a series of mutants with deletions in topoisomerase 3beta gene (top3beta) and an adjacent, previously uncharacterized gene CG15897, here named wuho (wh). Whereas top3beta truncation does not affect viability or fertility, wh null mutants display male sterile and female semi-sterile phenotypes. Furthermore, wh mutants can be fully rescued by wh transgenes, but not by top3beta transgenes, suggesting that the fertility phenotypes are caused by wh deletion. The alignment of WH protein sequence with other eukaryotic putative homologues shows they are evolutionarily conserved proteins with 5 WD40 repeats in the middle portion of the protein, and a bipartite nuclear localization signal at the carboxyl terminus. Yeast homologue with 5 WD40 repeats, Trm82, is the non-catalytic subunit of a tRNA methylase. Immunostaining shows that WH has the highest expression in hub cells, a niche for germline stem cells of testis. However, WH is not required for the maintenance of hub cells or the germline stem cells. In wh mutant males, spermatogenesis is arrested at the elongating stage of the developing spermatids, resulting in an absence of mature sperms in the seminal vesicles. The decreased fertility in wh mutant females is mostly due to defects in oogenesis. There are abnormal egg chambers present in the mutant females, in which the cystocytes fail to arrest their cell division at the fourth mitotic cycle, resulting in more than 16 cells in a single egg chamber. Additionally, these abnormal cystocytes do not undergo multiple rounds of endoreplication as the nurse cells do in a normal egg chamber. Therefore, the cytological analyses demonstrate that wh has a critical function in cellular differentiation for germline cells during gametogenesis.
Figures





Similar articles
-
WD40 protein Wuho controls germline homeostasis via TRIM-NHL tumor suppressor Mei-p26 in Drosophila.Development. 2020 Jan 15;147(2):dev182063. doi: 10.1242/dev.182063. Development. 2020. PMID: 31941704 Free PMC article.
-
Drosophila male and female germline stem cell niches require the nuclear lamina protein Otefin.Dev Biol. 2016 Jul 1;415(1):75-86. doi: 10.1016/j.ydbio.2016.05.001. Epub 2016 May 10. Dev Biol. 2016. PMID: 27174470 Free PMC article.
-
Soma-to-germline interactions during Drosophila oogenesis are influenced by dose-sensitive interactions between cut and the genes cappuccino, ovarian tumor and agnostic.Genetics. 1999 Sep;153(1):289-303. doi: 10.1093/genetics/153.1.289. Genetics. 1999. PMID: 10471713 Free PMC article.
-
Knockdown of RpL36 in testes impairs spermatogenesis in Drosophila melanogaster.J Exp Zool B Mol Dev Evol. 2021 Jul;336(5):417-430. doi: 10.1002/jez.b.23040. Epub 2021 Mar 18. J Exp Zool B Mol Dev Evol. 2021. PMID: 33734578
-
[Progress of recent studies on spermatogenesis genes and function regulations in Drosophila melanogaster].Sheng Li Ke Xue Jin Zhan. 2013 Apr;44(2):138-42. Sheng Li Ke Xue Jin Zhan. 2013. PMID: 23847927 Review. Chinese. No abstract available.
Cited by
-
Top3β is an RNA topoisomerase that works with fragile X syndrome protein to promote synapse formation.Nat Neurosci. 2013 Sep;16(9):1238-47. doi: 10.1038/nn.3479. Epub 2013 Aug 4. Nat Neurosci. 2013. PMID: 23912945 Free PMC article.
-
7-Methylguanosine Modifications in Transfer RNA (tRNA).Int J Mol Sci. 2018 Dec 17;19(12):4080. doi: 10.3390/ijms19124080. Int J Mol Sci. 2018. PMID: 30562954 Free PMC article. Review.
-
Topoisomerase 3β interacts with RNAi machinery to promote heterochromatin formation and transcriptional silencing in Drosophila.Nat Commun. 2018 Nov 23;9(1):4946. doi: 10.1038/s41467-018-07101-4. Nat Commun. 2018. PMID: 30470739 Free PMC article.
-
Wuho Is a New Member in Maintaining Genome Stability through its Interaction with Flap Endonuclease 1.PLoS Biol. 2016 Jan 11;14(1):e1002349. doi: 10.1371/journal.pbio.1002349. eCollection 2016 Jan. PLoS Biol. 2016. PMID: 26751069 Free PMC article.
-
Physiological significance of the two isoforms of initiator tRNAs in Escherichia coli.J Bacteriol. 2024 Sep 19;206(9):e0025124. doi: 10.1128/jb.00251-24. Epub 2024 Aug 22. J Bacteriol. 2024. PMID: 39171914
References
-
- Brower DL, Smith RJ, Wilcox M. Differentiation within the gonads of Drosophila revealed by immunofluorescence. J Embryol Exp Morphol. 1981;63:233–242. - PubMed
-
- Chen D, McKearin DM. A discrete transcriptional silencer in the bam gene determines asymmetric division of the Drosophila germline stem cell. Development. 2003;130:1159–1170. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

