Matrix metalloproteinase 2 (MMP2) and MMP9 secreted by erythropoietin-activated endothelial cells promote neural progenitor cell migration
- PMID: 16738242
- PMCID: PMC6675216
- DOI: 10.1523/JNEUROSCI.5380-05.2006
Matrix metalloproteinase 2 (MMP2) and MMP9 secreted by erythropoietin-activated endothelial cells promote neural progenitor cell migration
Abstract
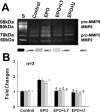
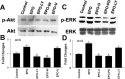
We investigated the hypothesis that endothelial cells activated by erythropoietin (EPO) promote the migration of neuroblasts. This hypothesis is based on observations in vivo that treatment of focal cerebral ischemia with EPO enhances the migration of neuroblasts to the ischemic boundary, a site containing activated endothelial cells and angiogenic microvasculature. To model the microenvironment within the ischemic boundary zone, we used a coculture system of mouse brain endothelial cells (MBECs) and neural progenitor cells derived from the subventricular zone of the adult mouse. Treatment of MBECs with recombinant human EPO (rhEPO) significantly increased secretion of matrix metalloproteinase 2 (MMP2) and MMP9. rhEPO-treated MBEC supernatant as conditioned medium significantly increased the migration of neural progenitor cells. Application of an MMP inhibitor abolished the supernatant-enhanced migration. Incubation of neurospheres alone with rhEPO failed to increase progenitor cell migration. rhEPO activated phosphatidylinositol 3-kinase/Akt (PI3K/Akt) and extracellular signal-regulated kinase (ERK1/2) in MBECs. Selective inhibition of the PI3K/Akt and ERK1/2 pathways significantly attenuated the rhEPO-induced MMP2 and MMP9, which suppressed neural progenitor cell migration promoted by the rhEPO-activated MBECs. Collectively, our data show that rhEPO-activated endothelial cells enhance neural progenitor cell migration by secreting MMP2 and MMP9 via the PI3K/Akt and ERK1/2 signaling pathways. These data demonstrate that activated endothelial cells can promote neural progenitor cell migration, and provide insight into the molecular mechanisms underlying the attraction of newly generated neurons to injured areas in brain.
Figures
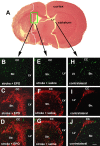
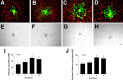
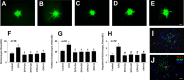
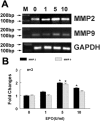
Similar articles
-
Neural progenitor cells treated with EPO induce angiogenesis through the production of VEGF.J Cereb Blood Flow Metab. 2008 Jul;28(7):1361-8. doi: 10.1038/jcbfm.2008.32. Epub 2008 Apr 16. J Cereb Blood Flow Metab. 2008. PMID: 18414495 Free PMC article.
-
Neurogenin 1 mediates erythropoietin enhanced differentiation of adult neural progenitor cells.J Cereb Blood Flow Metab. 2006 Apr;26(4):556-64. doi: 10.1038/sj.jcbfm.9600215. J Cereb Blood Flow Metab. 2006. PMID: 16136056
-
Erythropoietin-mediated protection of insect brain neurons involves JAK and STAT but not PI3K transduction pathways.Neuroscience. 2014 Jan 31;258:218-27. doi: 10.1016/j.neuroscience.2013.11.020. Epub 2013 Nov 21. Neuroscience. 2014. PMID: 24269933
-
Techniques and strategies to analyze neural progenitor cell migration.Curr Pharm Biotechnol. 2007 Jun;8(3):177-85. doi: 10.2174/138920107780906513. Curr Pharm Biotechnol. 2007. PMID: 17584090 Review.
-
MMP9: A Tough Target for Targeted Therapy for Cancer.Cancers (Basel). 2022 Apr 6;14(7):1847. doi: 10.3390/cancers14071847. Cancers (Basel). 2022. PMID: 35406619 Free PMC article. Review.
Cited by
-
The ERK1/2 Inhibitor U0126 Attenuates Diabetes-Induced Upregulation of MMP-9 and Biomarkers of Inflammation in the Retina.J Diabetes Res. 2013;2013:658548. doi: 10.1155/2013/658548. Epub 2013 Apr 10. J Diabetes Res. 2013. PMID: 23671886 Free PMC article.
-
MRI detects brain reorganization after human umbilical tissue-derived cells (hUTC) treatment of stroke in rat.PLoS One. 2012;7(8):e42845. doi: 10.1371/journal.pone.0042845. Epub 2012 Aug 10. PLoS One. 2012. PMID: 22900057 Free PMC article.
-
RGS16 regulated by let-7c-5p promotes glioma progression by activating PI3K-AKT pathway.Front Med. 2023 Feb;17(1):143-155. doi: 10.1007/s11684-022-0929-y. Epub 2022 Nov 22. Front Med. 2023. PMID: 36414916
-
Matrix Deformation with Ectopic Cells Induced by Rotational Motion in Bioengineered Neural Tissues.Ann Biomed Eng. 2020 Aug;48(8):2192-2203. doi: 10.1007/s10439-020-02561-6. Epub 2020 Jul 15. Ann Biomed Eng. 2020. PMID: 32671625 Free PMC article.
-
Nitric oxide donor upregulation of stromal cell-derived factor-1/chemokine (CXC motif) receptor 4 enhances bone marrow stromal cell migration into ischemic brain after stroke.Stem Cells. 2007 Nov;25(11):2777-85. doi: 10.1634/stemcells.2007-0169. Epub 2007 Jul 19. Stem Cells. 2007. PMID: 17641243 Free PMC article.
References
-
- Arcasoy MO, Jiang X (2005). Co-operative signalling mechanisms required for erythroid precursor expansion in response to erythropoietin and stem cell factor. Br J Haematol 130:121–129. - PubMed
-
- Arkell J, Jackson CJ (2003). Constitutive secretion of MMP9 by early-passage cultured human endothelial cells. Cell Biochem Funct 21:381–386. - PubMed
-
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O (2002). Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med 8:963–970. - PubMed
-
- Aye MM, Ma C, Lin H, Bower KA, Wiggins RC, Luo J (2004). Ethanol-induced in vitro invasion of breast cancer cells: the contribution of MMP-2 by fibroblasts. Int J Cancer 112:738–746. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
