A hybrid two-component system protein of a prominent human gut symbiont couples glycan sensing in vivo to carbohydrate metabolism
- PMID: 16735464
- PMCID: PMC1472243
- DOI: 10.1073/pnas.0603249103
A hybrid two-component system protein of a prominent human gut symbiont couples glycan sensing in vivo to carbohydrate metabolism
Abstract
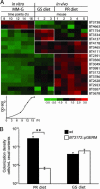
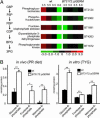
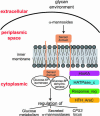
Bacteroides thetaiotaomicron is a prominent member of our normal adult intestinal microbial community and a useful model for studying the foundations of human-bacterial mutualism in our densely populated distal gut microbiota. A central question is how members of this microbiota sense nutrients and implement an appropriate metabolic response. B. thetaiotaomicron contains a large number of glycoside hydrolases not represented in our own proteome, plus a markedly expanded collection of hybrid two-component system (HTCS) proteins that incorporate all domains found in classical two-component environmental sensors into one polypeptide. To understand the role of HTCS in nutrient sensing, we used B. thetaiotaomicron GeneChips to characterize their expression in gnotobiotic mice consuming polysaccharide-rich or -deficient diets. One HTCS, BT3172, was selected for further analysis because it is induced in vivo by polysaccharides, and its absence reduces B. thetaiotaomicron fitness in polysaccharide-rich diet-fed mice. Functional genomic and biochemical analyses of WT and BT3172-deficient strains in vivo and in vitro disclosed that alpha-mannosides induce BT3172 expression, which in turn induces expression of secreted alpha-mannosidases. Yeast two-hybrid screens revealed that the cytoplasmic portion of BT3172's sensor domain serves as a scaffold for recruiting glucose-6-phosphate isomerase and dehydrogenase. These interactions are a unique feature of BT3172 and specific for the cytoplasmic face of its sensor domain. Loss of BT3172 reduces glycolytic pathway activity in vitro and in vivo. Thus, this HTCS functions as a metabolic reaction center, coupling nutrient sensing to dynamic regulation of monosaccharide metabolism. An expanded repertoire of HTCS proteins with diversified sensor domains may be one reason for B. thetaiotaomicron's success in our intestinal ecosystem.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures

Similar articles
-
Multifunctional nutrient-binding proteins adapt human symbiotic bacteria for glycan competition in the gut by separately promoting enhanced sensing and catalysis.mBio. 2014 Sep 9;5(5):e01441-14. doi: 10.1128/mBio.01441-14. mBio. 2014. PMID: 25205092 Free PMC article.
-
Genomic and metabolic studies of the impact of probiotics on a model gut symbiont and host.PLoS Biol. 2006 Nov;4(12):e413. doi: 10.1371/journal.pbio.0040413. PLoS Biol. 2006. PMID: 17132046 Free PMC article.
-
Glycan foraging in vivo by an intestine-adapted bacterial symbiont.Science. 2005 Mar 25;307(5717):1955-9. doi: 10.1126/science.1109051. Science. 2005. PMID: 15790854
-
How host-microbial interactions shape the nutrient environment of the mammalian intestine.Annu Rev Nutr. 2002;22:283-307. doi: 10.1146/annurev.nutr.22.011602.092259. Epub 2002 Apr 4. Annu Rev Nutr. 2002. PMID: 12055347 Review.
-
Navigating the Gut Buffet: Control of Polysaccharide Utilization in Bacteroides spp.Trends Microbiol. 2017 Dec;25(12):1005-1015. doi: 10.1016/j.tim.2017.06.009. Epub 2017 Jul 18. Trends Microbiol. 2017. PMID: 28733133 Review.
Cited by
-
The Prevention of Viral Infections: The Role of Intestinal Microbiota and Nutritional Factors.Nutrients. 2024 Jul 27;16(15):2445. doi: 10.3390/nu16152445. Nutrients. 2024. PMID: 39125326 Free PMC article. Review.
-
Glycosulfatase-Encoding Gene Cluster in Bifidobacterium breve UCC2003.Appl Environ Microbiol. 2016 Oct 27;82(22):6611-6623. doi: 10.1128/AEM.02022-16. Print 2016 Nov 15. Appl Environ Microbiol. 2016. PMID: 27590817 Free PMC article.
-
The lysine decarboxylase CadA protects Escherichia coli starved of phosphate against fermentation acids.J Bacteriol. 2007 Mar;189(6):2249-61. doi: 10.1128/JB.01306-06. Epub 2007 Jan 5. J Bacteriol. 2007. PMID: 17209032 Free PMC article.
-
Polysaccharides utilization in human gut bacterium Bacteroides thetaiotaomicron: comparative genomics reconstruction of metabolic and regulatory networks.BMC Genomics. 2013 Dec 12;14:873. doi: 10.1186/1471-2164-14-873. BMC Genomics. 2013. PMID: 24330590 Free PMC article.
-
A Master Regulator of Bacteroides thetaiotaomicron Gut Colonization Controls Carbohydrate Utilization and an Alternative Protein Synthesis Factor.mBio. 2020 Jan 28;11(1):e03221-19. doi: 10.1128/mBio.03221-19. mBio. 2020. PMID: 31992627 Free PMC article.
References
-
- Ley R. E., Peterson D. A, Gordon J. I. Cell. 2006;124:837–848. - PubMed
-
- Coutinho P. M., Henrissat B. In: Recent Advances in Carbohydrate Bioengineering. Gilbert H. J., Davies G., Henrissat B., Svensson B., editors. Cambridge, U.K.: R. Soc. Chem.; 1999. pp. 3–12.
-
- Sonnenburg J. L., Xu J., Leip D. D., Chen C. H., Westover B. P., Weatherford J., Buhler J. D, Gordon J. I. Science. 2005;307:1955–1959. - PubMed
-
- Backhed F., Ley R. E., Sonnenburg J. L., Peterson D. A., Gordon J. I. Science. 2005;307:1915–1920. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

