CD28 co-stimulation via tumour-specific chimaeric receptors induces an incomplete activation response in Epstein-Barr virus-specific effector memory T cells
- PMID: 16734614
- PMCID: PMC1941988
- DOI: 10.1111/j.1365-2249.2006.03095.x
CD28 co-stimulation via tumour-specific chimaeric receptors induces an incomplete activation response in Epstein-Barr virus-specific effector memory T cells
Abstract
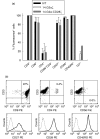
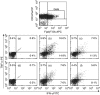
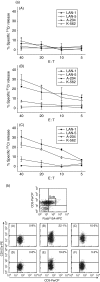
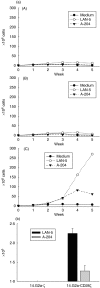
Expression of tumour antigen-specific chimaeric receptors in T lymphocytes can redirect their effector functions towards tumour cells. Integration of the signalling domains of the co-stimulatory molecule CD28 into chRec enhances antigen-specific proliferation of polyclonal human T cell populations. While CD28 plays an essential role in the priming of naive CD4(+) T cells, its contribution to effector memory T cell responses is controversial. We compared the function of the chRec with and without the CD28 co-stimulatory domain, expressing it in peripheral blood T cells or Epstein-Barr virus (EBV)-specific T cell lines. The chimaeric T cell receptors contain an extracellular single-chain antibody domain, to give specificity against the tumour ganglioside antigen G(D2). The transduced cytotoxic T lymphocytes (CTL) maintained their specificity for autologous EBV targets and their capacity to proliferate after stimulation with EBV-infected B cells. Intracellular cytokine staining demonstrated efficient and comparable antigen-specific interferon (IFN)-gamma secretion by CTL following engagement of both the native and the chimaeric receptor, independent of chimaeric CD28 signalling. Furthermore, tumour targets were lysed in an antigen-specific manner by both chRec. However, while antigen engagement by CD28 zeta chRec efficiently induced expansion of polyclonal peripheral blood lymphocytes in an antigen-dependent manner, CD28 signalling did not induce proliferation of EBV-CTL in response to antigen-expressing tumour cells. Thus, the co-stimulatory requirement for the efficient activation response of antigen-specific memory cells cannot be mimicked simply by combining CD28 and zeta signalling. The full potential of this highly cytolytic T cell population for adoptive immunotherapy of cancer requires further exploration of their co-stimulatory requirements.
Figures
Similar articles
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
[Construction of CD138-targeted chimeric antigen receptor- modified T cells and their effect in multiple myeloma therapy].Zhonghua Xue Ye Xue Za Zhi. 2024 May 14;45(5):436-444. doi: 10.3760/cma.j.cn121090-20240131-00047. Zhonghua Xue Ye Xue Za Zhi. 2024. PMID: 38964917 Free PMC article. Chinese.
-
2B4 (CD244) signaling via chimeric receptors costimulates tumor-antigen specific proliferation and in vitro expansion of human T cells.Cancer Immunol Immunother. 2009 Dec;58(12):1991-2001. doi: 10.1007/s00262-009-0704-9. Epub 2009 Apr 10. Cancer Immunol Immunother. 2009. PMID: 19360406 Free PMC article.
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
-
Antigen-specific T cells and autoimmunity.J Autoimmun. 2024 Sep;148:103303. doi: 10.1016/j.jaut.2024.103303. Epub 2024 Aug 13. J Autoimmun. 2024. PMID: 39141985 Review.
Cited by
-
Chimeric Antigen Receptor Signaling Domains Differentially Regulate Proliferation and Native T Cell Receptor Function in Virus-Specific T Cells.Front Med (Lausanne). 2018 Dec 11;5:343. doi: 10.3389/fmed.2018.00343. eCollection 2018. Front Med (Lausanne). 2018. PMID: 30619856 Free PMC article.
-
The ganglioside antigen G(D2) is surface-expressed in Ewing sarcoma and allows for MHC-independent immune targeting.Br J Cancer. 2012 Mar 13;106(6):1123-33. doi: 10.1038/bjc.2012.57. Epub 2012 Feb 28. Br J Cancer. 2012. PMID: 22374462 Free PMC article.
-
Preparing clinical grade Ag-specific T cells for adoptive immunotherapy trials.Cytotherapy. 2007;9(7):613-29. doi: 10.1080/14653240701650320. Cytotherapy. 2007. PMID: 17943498 Free PMC article. Review.
-
Ewing sarcoma dissemination and response to T-cell therapy in mice assessed by whole-body magnetic resonance imaging.Br J Cancer. 2013 Aug 6;109(3):658-66. doi: 10.1038/bjc.2013.356. Epub 2013 Jul 9. Br J Cancer. 2013. PMID: 23839490 Free PMC article.
-
EZH2 Inhibition in Ewing Sarcoma Upregulates GD2 Expression for Targeting with Gene-Modified T Cells.Mol Ther. 2019 May 8;27(5):933-946. doi: 10.1016/j.ymthe.2019.02.014. Epub 2019 Feb 23. Mol Ther. 2019. PMID: 30879952 Free PMC article.
References
-
- Rossig C, Brenner MK. Genetic modification of T lymphocytes for adoptive immunotherapy. Mol Ther. 2004;10:5–18. - PubMed
-
- Brentjens RJ, Latouche JB, Santos E, et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat Med. 2003;9:279–86. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

