Liver-specific deletion of acetyl-CoA carboxylase 1 reduces hepatic triglyceride accumulation without affecting glucose homeostasis
- PMID: 16717184
- PMCID: PMC1570106
- DOI: 10.1073/pnas.0603115103
Liver-specific deletion of acetyl-CoA carboxylase 1 reduces hepatic triglyceride accumulation without affecting glucose homeostasis
Abstract
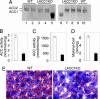
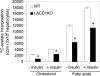
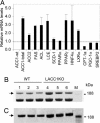
In animals, liver and white adipose are the main sites for the de novo fatty acid synthesis. Deletion of fatty acid synthase or acetyl-CoA carboxylase (ACC) 1 in mice resulted in embryonic lethality, indicating that the de novo fatty acid synthesis is essential for embryonic development. To understand the importance of de novo fatty acid synthesis and the role of ACC1-produced malonyl-CoA in adult mouse tissues, we generated liver-specific ACC1 knockout (LACC1KO) mice. LACC1KO mice have no obvious health problem under normal feeding conditions. Total ACC activity and malonyl-CoA levels were approximately 70-75% lower in liver of LACC1KO mice compared with that of the WT mice. In addition, the livers of LACC1KO mice accumulated 40-70% less triglycerides. Unexpectedly, when fed fat-free diet for 10 days, there was significant up-regulation of PPARgamma and several enzymes in the lipogenic pathway in the liver of LACC1KO mice compared with the WT mice. Despite the significant up-regulation of the lipogenic enzymes, including a >2-fold increase in fatty acid synthase mRNA, protein, and activity, there was significant decrease in the de novo fatty acid synthesis and triglyceride accumulation in the liver. However, there were no significant changes in blood glucose and fasting ketone body levels. Hence, reducing cytosolic malonyl-CoA and, therefore, the de novo fatty acid synthesis in the liver, does not affect fatty acid oxidation and glucose homeostasis under lipogenic conditions.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures

Similar articles
-
Hepatic de novo lipogenesis is present in liver-specific ACC1-deficient mice.Mol Cell Biol. 2007 Mar;27(5):1881-8. doi: 10.1128/MCB.01122-06. Epub 2007 Jan 8. Mol Cell Biol. 2007. PMID: 17210641 Free PMC article.
-
Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2.Science. 2001 Mar 30;291(5513):2613-6. doi: 10.1126/science.1056843. Science. 2001. PMID: 11283375
-
Acetyl-CoA carboxylase 2-/- mutant mice are protected against fatty liver under high-fat, high-carbohydrate dietary and de novo lipogenic conditions.J Biol Chem. 2012 Apr 6;287(15):12578-88. doi: 10.1074/jbc.M111.309559. Epub 2012 Feb 23. J Biol Chem. 2012. PMID: 22362781 Free PMC article.
-
Regulation of food intake and energy expenditure by hypothalamic malonyl-CoA.Int J Obes (Lond). 2008 Sep;32 Suppl 4:S49-54. doi: 10.1038/ijo.2008.123. Int J Obes (Lond). 2008. PMID: 18719599 Review.
-
Malonyl-CoA, a key signaling molecule in mammalian cells.Annu Rev Nutr. 2008;28:253-72. doi: 10.1146/annurev.nutr.28.061807.155434. Annu Rev Nutr. 2008. PMID: 18598135 Review.
Cited by
-
Ganoderma lucidum culture supplement ameliorates dyslipidemia and reduces visceral fat accumulation in type 2 diabetic rats.Mycology. 2020 Mar 23;12(2):94-104. doi: 10.1080/21501203.2020.1740409. Mycology. 2020. PMID: 34026301 Free PMC article.
-
Oligonol suppresses lipid accumulation and improves insulin resistance in a palmitate-induced in HepG2 hepatocytes as a cellular steatosis model.BMC Complement Altern Med. 2015 Jun 16;15:185. doi: 10.1186/s12906-015-0709-1. BMC Complement Altern Med. 2015. PMID: 26077338 Free PMC article.
-
AMPK-ACC signaling modulates platelet phospholipids and potentiates thrombus formation.Blood. 2018 Sep 13;132(11):1180-1192. doi: 10.1182/blood-2018-02-831503. Epub 2018 Jul 17. Blood. 2018. PMID: 30018077 Free PMC article.
-
Loss of progesterone receptor membrane component 1 promotes hepatic steatosis via the induced de novo lipogenesis.Sci Rep. 2018 Oct 24;8(1):15711. doi: 10.1038/s41598-018-34148-6. Sci Rep. 2018. PMID: 30356113 Free PMC article.
-
Effects of Low Protein-High Carbohydrate Diet during Early and Late Pregnancy on Respiratory Quotient and Visceral Adiposity.Oxid Med Cell Longev. 2022 Apr 7;2022:3878581. doi: 10.1155/2022/3878581. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35432727 Free PMC article.
References
-
- Wakil S. J. Biochemistry. 1989;28:4523–4530. - PubMed
-
- Smith S. FASEB J. 1994;8:1248–1259. - PubMed
-
- Hillgartner F. B., Salati L. M., Goodridge A. G. Physiol. Rev. 1995;75:47–75. - PubMed
-
- Girard J., Ferre P., Foufelle F. Annu. Rev. Nutr. 1997;17:325–352. - PubMed
-
- Wakil S. J., Stoops J. K., Joshi V. C. Annu. Rev. Biochem. 1983;52:537–579. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

