Reprogramming of antiviral T cells prevents inactivation and restores T cell activity during persistent viral infection
- PMID: 16710479
- PMCID: PMC1462941
- DOI: 10.1172/JCI26856
Reprogramming of antiviral T cells prevents inactivation and restores T cell activity during persistent viral infection
Abstract
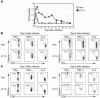
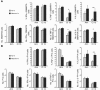
Failure to clear persistent viral infections results from the early loss of T cell activity. A pertinent question is whether the immune response is programmed to fail or if nonresponsive T cells can specifically be fixed to eliminate infection. Although evidence indicates that T cell expansion is permanently programmed during the initial priming events, the mechanisms that determine the acquisition of T cell function are less clear. Herein we show that in contrast to expansion, the functional programming of T cell effector and memory responses in vivo in mice is not hardwired during priming but is alterable and responsive to continuous instruction from the antigenic environment. As a direct consequence, dysfunctional T cells can be functionally reactivated during persistent infection even after an initial program of inactivation has been instituted. We also show that early therapeutic reductions in viral replication facilitate the preservation of antiviral CD4+ T cell activity, enabling the long-term control of viral replication. Thus, dysfunctional antiviral T cells can regain activity, providing a basis for future therapeutic strategies to treat persistent viral infections.
Figures




Similar articles
-
Differential role of IL-2R signaling for CD8+ T cell responses in acute and chronic viral infections.Eur J Immunol. 2007 Jun;37(6):1502-12. doi: 10.1002/eji.200637023. Eur J Immunol. 2007. PMID: 17492805
-
Principles for adoptive T cell therapy of human viral diseases.Annu Rev Immunol. 1995;13:545-86. doi: 10.1146/annurev.iy.13.040195.002553. Annu Rev Immunol. 1995. PMID: 7612234 Review.
-
Control of acute cutaneous herpes simplex virus infection: T cell-mediated viral clearance is dependent upon interferon-gamma (IFN-gamma).Virology. 1994 Jul;202(1):76-88. doi: 10.1006/viro.1994.1324. Virology. 1994. PMID: 7912023
-
Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets.Proc Natl Acad Sci U S A. 2004 Apr 13;101(15):5610-5. doi: 10.1073/pnas.0308054101. Epub 2004 Mar 25. Proc Natl Acad Sci U S A. 2004. PMID: 15044705 Free PMC article.
-
Noncytolytic control of viral infections by the innate and adaptive immune response.Annu Rev Immunol. 2001;19:65-91. doi: 10.1146/annurev.immunol.19.1.65. Annu Rev Immunol. 2001. PMID: 11244031 Review.
Cited by
-
Population dynamics and gene regulation of T cells in response to chronic antigen stimulation.Int Immunol. 2023 Feb 11;35(2):67-77. doi: 10.1093/intimm/dxac050. Int Immunol. 2023. PMID: 36334059 Free PMC article. Review.
-
Dynamics of regulatory T-cells during pregnancy: effect of HIV infection and correlations with other immune parameters.PLoS One. 2011;6(11):e28172. doi: 10.1371/journal.pone.0028172. Epub 2011 Nov 29. PLoS One. 2011. PMID: 22140535 Free PMC article.
-
Pronounced virus-dependent activation drives exhaustion but sustains IFN-γ transcript levels.J Immunol. 2010 Sep 15;185(6):3643-51. doi: 10.4049/jimmunol.1000841. Epub 2010 Aug 18. J Immunol. 2010. PMID: 20720198 Free PMC article.
-
Chronic antigen stimulation alone is sufficient to drive CD8+ T cell exhaustion.J Immunol. 2009 Jun 1;182(11):6697-708. doi: 10.4049/jimmunol.0800997. J Immunol. 2009. PMID: 19454664 Free PMC article.
-
Varicella-Zoster Virus-Specific Cellular Immune Responses to the Live Attenuated Zoster Vaccine in Young and Older Adults.J Immunol. 2017 Jul 15;199(2):604-612. doi: 10.4049/jimmunol.1700290. Epub 2017 Jun 12. J Immunol. 2017. PMID: 28607114 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

