Fgf9 and Wnt4 act as antagonistic signals to regulate mammalian sex determination
- PMID: 16700629
- PMCID: PMC1463023
- DOI: 10.1371/journal.pbio.0040187
Fgf9 and Wnt4 act as antagonistic signals to regulate mammalian sex determination
Abstract
The genes encoding members of the wingless-related MMTV integration site (WNT) and fibroblast growth factor (FGF) families coordinate growth, morphogenesis, and differentiation in many fields of cells during development. In the mouse, Fgf9 and Wnt4 are expressed in gonads of both sexes prior to sex determination. Loss of Fgf9 leads to XY sex reversal, whereas loss of Wnt4 results in partial testis development in XX gonads. However, the relationship between these signals and the male sex-determining gene, Sry, was unknown. We show through gain- and loss-of-function experiments that fibroblast growth factor 9 (FGF9) and WNT4 act as opposing signals to regulate sex determination. In the mouse XY gonad, Sry normally initiates a feed-forward loop between Sox9 and Fgf9, which up-regulates Fgf9 and represses Wnt4 to establish the testis pathway. Surprisingly, loss of Wnt4 in XX gonads is sufficient to up-regulate Fgf9 and Sox9 in the absence of Sry. These data suggest that the fate of the gonad is controlled by antagonism between Fgf9 and Wnt4. The role of the male sex-determining switch--Sry in the case of mammals--is to tip the balance between these underlying patterning signals. In principle, sex determination in other vertebrates may operate through any switch that introduces an imbalance between these two signaling pathways.
Figures
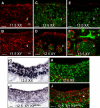
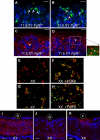
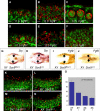

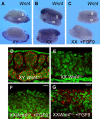
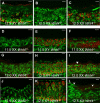
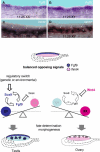
Comment in
-
Male or female? It depends on the dose.PLoS Biol. 2006 Jun;4(6):e211. doi: 10.1371/journal.pbio.0040211. Epub 2006 May 23. PLoS Biol. 2006. PMID: 20076594 Free PMC article. No abstract available.
Similar articles
-
FGF9 is a downstream target of SRY and sufficient to determine male sex fate in ex vivo XX gonad culture.Biol Reprod. 2020 Dec 1;103(6):1300-1313. doi: 10.1093/biolre/ioaa154. Biol Reprod. 2020. PMID: 32886743
-
A critical time window of Sry action in gonadal sex determination in mice.Development. 2009 Jan;136(1):129-38. doi: 10.1242/dev.029587. Epub 2008 Nov 26. Development. 2009. PMID: 19036799
-
Testis development requires the repression of Wnt4 by Fgf signaling.Dev Biol. 2012 Oct 1;370(1):24-32. doi: 10.1016/j.ydbio.2012.06.009. Epub 2012 Jun 15. Dev Biol. 2012. PMID: 22705479 Free PMC article.
-
Balancing the bipotential gonad between alternative organ fates: a new perspective on an old problem.Dev Dyn. 2006 Sep;235(9):2292-300. doi: 10.1002/dvdy.20894. Dev Dyn. 2006. PMID: 16881057 Review.
-
SRY and the standoff in sex determination.Mol Endocrinol. 2008 Jan;22(1):1-9. doi: 10.1210/me.2007-0250. Epub 2007 Jul 31. Mol Endocrinol. 2008. PMID: 17666585 Free PMC article. Review.
Cited by
-
Nodal/activin signaling promotes male germ cell fate and suppresses female programming in somatic cells.Development. 2013 Jan 15;140(2):291-300. doi: 10.1242/dev.087882. Epub 2012 Dec 5. Development. 2013. PMID: 23221368 Free PMC article.
-
Synergistic effect of SRY and its direct target, WDR5, on Sox9 expression.PLoS One. 2012;7(4):e34327. doi: 10.1371/journal.pone.0034327. Epub 2012 Apr 16. PLoS One. 2012. PMID: 22523547 Free PMC article.
-
Establishing and maintaining fertility: the importance of cell cycle arrest.Genes Dev. 2021 May 1;35(9-10):619-634. doi: 10.1101/gad.348151.120. Epub 2021 Apr 22. Genes Dev. 2021. PMID: 33888561 Free PMC article. Review.
-
Structural dynamics and inhibitor searching for Wnt-4 protein using comparative computational studies.Drug Des Devel Ther. 2015 Apr 29;9:2449-61. doi: 10.2147/DDDT.S79784. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 25995617 Free PMC article.
-
The Important Role of Sex-Related Sox Family Genes in the Sex Reversal of the Chinese Soft-Shelled Turtle (Pelodiscus sinensis).Biology (Basel). 2022 Jan 6;11(1):83. doi: 10.3390/biology11010083. Biology (Basel). 2022. PMID: 35053081 Free PMC article.
References
-
- Eicher EM, Washburn LL. Genetic control of primary sex determination in mice. Annu Rev Genet. 1986;20:327–360. - PubMed
-
- Albrecht KH, Eicher EM. Evidence that Sry is expressed in pre-Sertoli cells and Sertoli and granulosa cells have a common precursor . Dev Biol. 2001;240:92–107. - PubMed
-
- Hoyle C, Narvaez V, Alldus G, Lovell-Badge R, Swain A. Dax1 expression is dependent on steroidogenic factor 1 in the developing gonad . Mol Endocrinol. 2002;16:747–756. - PubMed
-
- Swain A, Narvaez V, Burgoyne P, Camerino G, Lovell-Badge R. Dax1 antagonizes Sry action in mammalian sex determination . Nature. 1998;391:761–767. - PubMed
-
- Morais da Silva S, Hacker A, Harley V, Goodfellow P, Swain A, et al. Sox9 expression during gonadal development implies a conserved role for the gene in testis differentiation in mammals and birds . Nat Genet. 1996;14:62–68. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

