IL-15 induces CD4 effector memory T cell production and tissue emigration in nonhuman primates
- PMID: 16691294
- PMCID: PMC1459071
- DOI: 10.1172/JCI27564
IL-15 induces CD4 effector memory T cell production and tissue emigration in nonhuman primates
Abstract
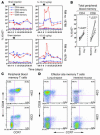
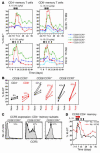
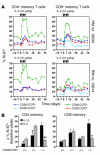
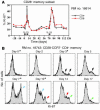
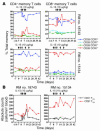
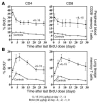
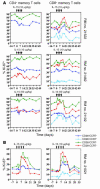
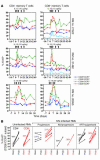
HIV infection selectively targets CD4+ effector memory T (T EM) cells, resulting in dramatic depletion of CD4+ T cells in mucosal effector sites in early infection. Regeneration of the T EM cell compartment is slow and incomplete, even when viral replication is controlled by antiretroviral therapy (ART). Here, we demonstrate that IL-15 dramatically increases in vivo proliferation of rhesus macaque (RM) CD4+ and CD8+ T EM cells with little effect on the naive or central memory T (T CM) cell subsets, a response pattern that is quite distinct from that of either IL-2 or IL-7. T EM cells produced in response to IL-15 did not accumulate in blood. Rather, 5-bromo-2'-deoxyuridine (BrdU) labeling studies suggest that many of these cells rapidly disperse to extralymphoid effector sites, where they manifest (slow) decay kinetics indistinguishable from that of untreated controls. In RMs with uncontrolled SIV infection and highly activated immune systems, IL-15 did not significantly increase CD4+ T EM cell proliferation, but with virologic control and concomitant reduction in immune activation by ART, IL-15 responsiveness was again observed. These data suggest that therapeutic use of IL-15 in the setting of ART might facilitate specific restoration of the CD4 + T cell compartment that is the primary target of HIV with less risk of exhausting precursor T cell compartments or generating potentially deleterious regulatory subsets.
Figures

Similar articles
-
Profound CD4+/CCR5+ T cell expansion is induced by CD8+ lymphocyte depletion but does not account for accelerated SIV pathogenesis.J Exp Med. 2009 Jul 6;206(7):1575-88. doi: 10.1084/jem.20090356. Epub 2009 Jun 22. J Exp Med. 2009. PMID: 19546246 Free PMC article.
-
Initiation of Antiretroviral Therapy Restores CD4+ T Memory Stem Cell Homeostasis in Simian Immunodeficiency Virus-Infected Macaques.J Virol. 2016 Jul 11;90(15):6699-6708. doi: 10.1128/JVI.00492-16. Print 2016 Aug 1. J Virol. 2016. PMID: 27170752 Free PMC article.
-
Simian immunodeficiency virus infection induces severe loss of intestinal central memory T cells which impairs CD4+ T-cell restoration during antiretroviral therapy.J Med Primatol. 2007 Aug;36(4-5):219-27. doi: 10.1111/j.1600-0684.2007.00239.x. J Med Primatol. 2007. PMID: 17669210
-
Therapeutic Potential of IL-15 and N-803 in HIV/SIV Infection.Viruses. 2021 Sep 2;13(9):1750. doi: 10.3390/v13091750. Viruses. 2021. PMID: 34578331 Free PMC article. Review.
-
HIV Persistence in Adipose Tissue Reservoirs.Curr HIV/AIDS Rep. 2018 Feb;15(1):60-71. doi: 10.1007/s11904-018-0378-z. Curr HIV/AIDS Rep. 2018. PMID: 29423731 Free PMC article. Review.
Cited by
-
Proliferation-linked apoptosis of adoptively transferred T cells after IL-15 administration in macaques.PLoS One. 2013;8(2):e56268. doi: 10.1371/journal.pone.0056268. Epub 2013 Feb 13. PLoS One. 2013. PMID: 23418547 Free PMC article.
-
Peptide-based identification of functional motifs and their binding partners.J Vis Exp. 2013 Jun 30;(76):50362. doi: 10.3791/50362. J Vis Exp. 2013. PMID: 23852082 Free PMC article.
-
Sustained viremia suppression by SHIVSF162P3CN-recalled effector-memory CD8+ T cells after PD1-based vaccination.PLoS Pathog. 2021 Jun 14;17(6):e1009647. doi: 10.1371/journal.ppat.1009647. eCollection 2021 Jun. PLoS Pathog. 2021. PMID: 34125864 Free PMC article.
-
The Frequency of Vaccine-Induced T-Cell Responses Does Not Predict the Rate of Acquisition after Repeated Intrarectal SIVmac239 Challenges in Mamu-B*08+ Rhesus Macaques.J Virol. 2019 Feb 19;93(5):e01626-18. doi: 10.1128/JVI.01626-18. Print 2019 Mar 1. J Virol. 2019. PMID: 30541854 Free PMC article.
-
Utilizing IL-12, IL-15 and IL-7 as Mucosal Vaccine Adjuvants.Lett Drug Des Discov. 2006;3(8):586-592. doi: 10.2174/157018006778194655. Lett Drug Des Discov. 2006. PMID: 17496983 Free PMC article.
References
-
- Douek D.C., Picker L.J., Koup R.A. T cell dynamics in HIV-1 infection. Annu. Rev. Immunol. 2003;21:265–304. - PubMed
-
- Veazey R., Lackner A. The mucosal immune system and HIV-1 infection. AIDS Rev. 2003;5:245–252. - PubMed
-
- Mehandru S., Tenner-Racz K., Racz P., Markowitz M. The gastrointestinal tract is critical to the pathogenesis of acute HIV-1 infection. . J. Allergy Clin. Immunol. 2005;116:419–422. - PubMed
-
- Sallusto F., Geginat J., Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 2004;22:745–763. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

