MR imaging of relapsing multiple sclerosis patients using ultra-small-particle iron oxide and compared with gadolinium
- PMID: 16687532
- PMCID: PMC7975759
MR imaging of relapsing multiple sclerosis patients using ultra-small-particle iron oxide and compared with gadolinium
Abstract
Background and purpose: Inflammatory multiple sclerosis (MS) lesions are characterized by microglia activation and infiltration of T cells, B cells, and macrophages across the blood-brain barrier (BBB). In the experimental autoimmune encephalomyelitis (EAE) rat model of MS, previous MR imaging investigations with a new contrast agent ultra-small-particle iron oxide (USPIO) that accumulates in phagocytic cells revealed in vivo the presence of macrophage brain infiltration. The goal of this study was to characterize MS lesions with the use of this contrast agent.
Methods: A prospective MR imaging study of 10 patients with MS in acute relapses was achieved by using USPIO and gadolinium.
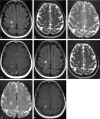
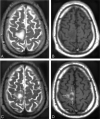
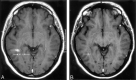
Results: Twenty-four hours after USPIO injection, 33 acute MS lesions in 9 patients showed USPIO uptake. Lesions were seen as high signal intensities on T1-weighted images and low signal intensities on T2-weighted images. Gadolinium enhancement was seen in 31 of these lesions in 7 patients. These 7 patients presented 24 gadolinium-enhanced lesions that did not enhance with USPIO. Two patients showed USPIO-enhanced lesions but no gadolinium-enhanced lesions.
Conclusion: Taken together with earlier findings obtained in experimental models or in human stroke, the visualization of macrophage activity in vivo with USPIO characterize a distinct cellular and inflammatory event of the dynamic process of MS lesion formation. The macrophage activity information obtained with USPIO is distinct and complementary to the increased BBB permeability seen with gadolinium.
Figures
Similar articles
-
Comparison of ultrasmall particles of iron oxide (USPIO)-enhanced T2-weighted, conventional T2-weighted, and gadolinium-enhanced T1-weighted MR images in rats with experimental autoimmune encephalomyelitis.AJNR Am J Neuroradiol. 1999 Feb;20(2):223-7. AJNR Am J Neuroradiol. 1999. PMID: 10094342 Free PMC article.
-
Assessment of disease activity in multiple sclerosis phenotypes with combined gadolinium- and superparamagnetic iron oxide-enhanced MR imaging.Radiology. 2012 Jul;264(1):225-33. doi: 10.1148/radiol.12111416. Radiology. 2012. PMID: 22723563 Clinical Trial.
-
Pluriformity of inflammation in multiple sclerosis shown by ultra-small iron oxide particle enhancement.Brain. 2008 Mar;131(Pt 3):800-7. doi: 10.1093/brain/awn009. Epub 2008 Feb 1. Brain. 2008. PMID: 18245785 Clinical Trial.
-
Macrophage imaging in central nervous system and in carotid atherosclerotic plaque using ultrasmall superparamagnetic iron oxide in magnetic resonance imaging.Invest Radiol. 2004 Oct;39(10):619-25. doi: 10.1097/01.rli.0000135980.08491.33. Invest Radiol. 2004. PMID: 15377941 Review.
-
[Iron-oxide-enhanced MR imaging of inflammatory atherosclerotic lesions: overview of experimental and initial clinical results].Rofo. 2003 Apr;175(4):469-76. doi: 10.1055/s-2003-38434. Rofo. 2003. PMID: 12677500 Review. German.
Cited by
-
Enhanced cellular uptake of aminosilane-coated superparamagnetic iron oxide nanoparticles in mammalian cell lines.Int J Nanomedicine. 2012;7:953-64. doi: 10.2147/IJN.S28316. Epub 2012 Feb 21. Int J Nanomedicine. 2012. PMID: 22393292 Free PMC article.
-
Anti-IL-17A treatment reduces clinical score and VCAM-1 expression detected by in vivo magnetic resonance imaging in chronic relapsing EAE ABH mice.Am J Pathol. 2013 Jun;182(6):2071-81. doi: 10.1016/j.ajpath.2013.02.029. Epub 2013 Apr 16. Am J Pathol. 2013. PMID: 23602647 Free PMC article.
-
Superparamagnetic iron oxide nanoparticles: diagnostic magnetic resonance imaging and potential therapeutic applications in neurooncology and central nervous system inflammatory pathologies, a review.J Cereb Blood Flow Metab. 2010 Jan;30(1):15-35. doi: 10.1038/jcbfm.2009.192. Epub 2009 Sep 16. J Cereb Blood Flow Metab. 2010. PMID: 19756021 Free PMC article. Review.
-
Cell tracking technologies for acute ischemic brain injury.J Cereb Blood Flow Metab. 2015 Jul;35(7):1090-9. doi: 10.1038/jcbfm.2015.93. Epub 2015 May 13. J Cereb Blood Flow Metab. 2015. PMID: 25966948 Free PMC article. Review.
-
Magnetic resonance imaging of blood brain/nerve barrier dysfunction and leukocyte infiltration: closely related or discordant?Front Neurol. 2012 Dec 21;3:178. doi: 10.3389/fneur.2012.00178. eCollection 2012. Front Neurol. 2012. PMID: 23267343 Free PMC article.
References
-
- Raivich G, Banati R. Brain microglia and blood-derived macrophages: molecular profiles and functional roles in multiple sclerosis and animal models of autoimmune demyelinating disease. Brain Res Brain Res Rev 2004;46:261–81 - PubMed
-
- Kornek B, Lassmann H. Neuropathology of multiple sclerosis—new concepts. Brain Res Bull 2003;61:321–26 - PubMed
-
- Lucchinetti C, Brück W, Noseworthy J. Multiple sclerosis: recent developments in neuropathology, pathogenesis, magnetic resonance imaging studies and treatment. Curr Opin Neurol 2001;14:259–69 - PubMed
-
- Noseworthy JH, Lucchinetti C, Rodriguez M, et al. Multiple sclerosis. N Engl J Med 2000;343:938–52 - PubMed
-
- Brück W, Porada P, Poser S, et al. Monocyte/macrophage differentiation in early multiple sclerosis lesions. Ann Neurol 1995;38:788–96 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
