Opposing effects of the UV lesion repair protein XPA and UV bypass polymerase eta on ATR checkpoint signaling
- PMID: 16675950
- PMCID: PMC1478198
- DOI: 10.1038/sj.emboj.7601123
Opposing effects of the UV lesion repair protein XPA and UV bypass polymerase eta on ATR checkpoint signaling
Erratum in
- EMBO J. 2006 Oct 18;25(20):5036
Abstract
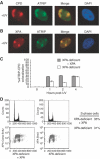
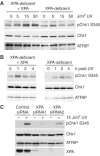
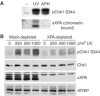
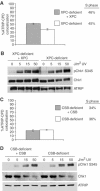
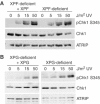
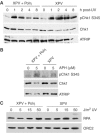
An essential component of the ATR (ataxia telangiectasia-mutated and Rad3-related)-activating structure is single-stranded DNA. It has been suggested that nucleotide excision repair (NER) can lead to activation of ATR by generating such a signal, and in yeast, DNA damage processing through the NER pathway is necessary for checkpoint activation during G1. We show here that ultraviolet (UV) radiation-induced ATR signaling is compromised in XPA-deficient human cells during S phase, as shown by defects in ATRIP (ATR-interacting protein) translocation to sites of UV damage, UV-induced phosphorylation of Chk1 and UV-induced replication protein A phosphorylation and chromatin binding. However, ATR signaling was not compromised in XPC-, CSB-, XPF- and XPG-deficient cells. These results indicate that damage processing is not necessary for ATR-mediated S-phase checkpoint activation and that the lesion recognition function of XPA may be sufficient. In contrast, XP-V cells deficient in the UV bypass polymerase eta exhibited enhanced ATR signaling. Taken together, these results suggest that lesion bypass and not lesion repair may raise the level of UV damage that can be tolerated before checkpoint activation, and that XPA plays a critical role in this activation.
Figures


Similar articles
-
Phosphorylation of nucleotide excision repair factor xeroderma pigmentosum group A by ataxia telangiectasia mutated and Rad3-related-dependent checkpoint pathway promotes cell survival in response to UV irradiation.Cancer Res. 2006 Mar 15;66(6):2997-3005. doi: 10.1158/0008-5472.CAN-05-3403. Cancer Res. 2006. PMID: 16540648 Free PMC article.
-
Checkpoint kinase ATR promotes nucleotide excision repair of UV-induced DNA damage via physical interaction with xeroderma pigmentosum group A.J Biol Chem. 2009 Sep 4;284(36):24213-22. doi: 10.1074/jbc.M109.000745. Epub 2009 Jul 8. J Biol Chem. 2009. PMID: 19586908 Free PMC article.
-
ATR-dependent checkpoint modulates XPA nuclear import in response to UV irradiation.Oncogene. 2007 Feb 1;26(5):757-64. doi: 10.1038/sj.onc.1209828. Epub 2006 Jul 24. Oncogene. 2007. PMID: 16862173 Free PMC article.
-
Xeroderma Pigmentosa Group A (XPA), Nucleotide Excision Repair and Regulation by ATR in Response to Ultraviolet Irradiation.Adv Exp Med Biol. 2017;996:41-54. doi: 10.1007/978-3-319-56017-5_4. Adv Exp Med Biol. 2017. PMID: 29124689 Free PMC article. Review.
-
The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and cancer.Adv Cancer Res. 2010;108:73-112. doi: 10.1016/B978-0-12-380888-2.00003-0. Adv Cancer Res. 2010. PMID: 21034966 Review.
Cited by
-
Non-transcriptional Function of FOXO1/DAF-16 Contributes to Translesion DNA Synthesis.Mol Cell Biol. 2016 Nov;36(21):2755-2766. doi: 10.1128/MCB.00265-16. Epub 2016 Aug 22. Mol Cell Biol. 2016. PMID: 27550812 Free PMC article.
-
Coupling of human DNA excision repair and the DNA damage checkpoint in a defined in vitro system.J Biol Chem. 2014 Feb 21;289(8):5074-82. doi: 10.1074/jbc.M113.542787. Epub 2014 Jan 8. J Biol Chem. 2014. PMID: 24403078 Free PMC article.
-
Chk2-dependent phosphorylation of XRCC1 in the DNA damage response promotes base excision repair.EMBO J. 2008 Dec 3;27(23):3140-50. doi: 10.1038/emboj.2008.229. Epub 2008 Oct 30. EMBO J. 2008. PMID: 18971944 Free PMC article.
-
Transcriptional inhibition and mutagenesis induced by N-nitroso compound-derived carboxymethylated thymidine adducts in DNA.Nucleic Acids Res. 2015 Jan;43(2):1012-8. doi: 10.1093/nar/gku1391. Epub 2015 Jan 8. Nucleic Acids Res. 2015. PMID: 25572317 Free PMC article.
-
Molecular mechanisms involved in initiation of the DNA damage response.Mol Cell Oncol. 2015 Jan 23;2(1):e970065. doi: 10.4161/23723548.2014.970065. eCollection 2015 Jan-Mar. Mol Cell Oncol. 2015. PMID: 27308403 Free PMC article. Review.
References
-
- Abraham RT (2001) Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev 15: 2177–2196 - PubMed
-
- Adimoolam S, Ford JM (2003) p53 and regulation of DNA damage recognition during nucleotide excision repair. DNA Repair (Amst) 2: 947–954 - PubMed
-
- Bakkenist CJ, Kastan MB (2004) Initiating cellular stress responses. Cell 118: 9–17 - PubMed
-
- Barr SM, Leung CG, Chang EE, Cimprich KA (2003) ATR kinase activity regulates the intranuclear translocation of ATR and RPA following ionizing radiation. Curr Biol 13: 1047–1051 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

