A sphingosine-1-phosphate-activated calcium channel controlling vascular smooth muscle cell motility
- PMID: 16675717
- PMCID: PMC2648505
- DOI: 10.1161/01.RES.0000225284.36490.a2
A sphingosine-1-phosphate-activated calcium channel controlling vascular smooth muscle cell motility
Abstract
In a screen of potential lipid regulators of transient receptor potential (TRP) channels, we identified sphingosine-1-phosphate (S1P) as an activator of TRPC5. We explored the relevance to vascular biology because S1P is a key cardiovascular signaling molecule. TRPC5 is expressed in smooth muscle cells of human vein along with TRPC1, which forms a complex with TRPC5. Importantly, S1P also activates the TRPC5-TRPC1 heteromultimeric channel. Because TRPC channels are linked to neuronal growth cone extension, we considered a related concept for smooth muscle. We find S1P stimulates smooth muscle cell motility, and that this is inhibited by E3-targeted anti-TRPC5 antibody. Ion permeation involving TRPC5 is crucial because S1P-evoked motility is also suppressed by the channel blocker 2-aminoethoxydiphenyl borate or a TRPC5 ion-pore mutant. S1P acts on TRPC5 via two mechanisms, one extracellular and one intracellular, consistent with its bipolar signaling functions. The extracellular effect appears to have a primary role in S1P-evoked cell motility. The data suggest S1P sensing by TRPC5 calcium channel is a mechanism contributing to vascular smooth muscle adaptation.
Figures
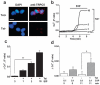
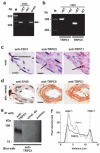
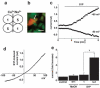
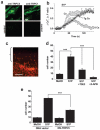
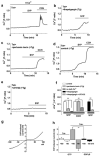
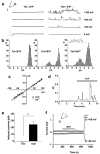
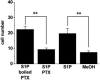
Comment in
-
Sphingolipids and transient receptor potential channels: evolutionarily ancient families now joined.Circ Res. 2006 Jun 9;98(11):1347-8. doi: 10.1161/01.RES.0000228464.97010.ee. Circ Res. 2006. PMID: 16763170 Review. No abstract available.
Similar articles
-
GVI phospholipase A2 role in the stimulatory effect of sphingosine-1-phosphate on TRPC5 cationic channels.Cell Calcium. 2011 Oct;50(4):343-50. doi: 10.1016/j.ceca.2011.06.003. Epub 2011 Jul 13. Cell Calcium. 2011. PMID: 21742378 Free PMC article.
-
Stereo-selective inhibition of transient receptor potential TRPC5 cation channels by neuroactive steroids.Br J Pharmacol. 2011 Apr;162(7):1509-20. doi: 10.1111/j.1476-5381.2010.01136.x. Br J Pharmacol. 2011. PMID: 21108630 Free PMC article.
-
S1P activates store-operated calcium entry via receptor- and non-receptor-mediated pathways in vascular smooth muscle cells.Am J Physiol Cell Physiol. 2011 Apr;300(4):C919-26. doi: 10.1152/ajpcell.00350.2010. Epub 2011 Jan 26. Am J Physiol Cell Physiol. 2011. PMID: 21270296 Free PMC article.
-
Bipolar phospholipid sensing by TRPC5 calcium channel.Biochem Soc Trans. 2007 Feb;35(Pt 1):101-4. doi: 10.1042/BST0350101. Biochem Soc Trans. 2007. PMID: 17233612 Review.
-
Canonical transient receptor potential 5.Handb Exp Pharmacol. 2007;(179):109-23. doi: 10.1007/978-3-540-34891-7_6. Handb Exp Pharmacol. 2007. PMID: 17217053 Review.
Cited by
-
Regulation of Membrane Calcium Transport Proteins by the Surrounding Lipid Environment.Biomolecules. 2019 Sep 20;9(10):513. doi: 10.3390/biom9100513. Biomolecules. 2019. PMID: 31547139 Free PMC article. Review.
-
G protein-coupled receptor signalling potentiates the osmo-mechanical activation of TRPC5 channels.Pflugers Arch. 2014 Aug;466(8):1635-46. doi: 10.1007/s00424-013-1392-z. Epub 2013 Nov 1. Pflugers Arch. 2014. PMID: 24177920
-
Permissive Modulation of Sphingosine-1-Phosphate-Enhanced Intracellular Calcium on BKCa Channel of Chromaffin Cells.Int J Mol Sci. 2021 Feb 22;22(4):2175. doi: 10.3390/ijms22042175. Int J Mol Sci. 2021. PMID: 33671654 Free PMC article.
-
The phospholipid-binding protein SESTD1 is a novel regulator of the transient receptor potential channels TRPC4 and TRPC5.J Biol Chem. 2010 Apr 16;285(16):12426-34. doi: 10.1074/jbc.M109.068304. Epub 2010 Feb 17. J Biol Chem. 2010. PMID: 20164195 Free PMC article.
-
Lysophosphatidyl choline modulates mechanosensitive L-type Ca2+ current in circular smooth muscle cells from human jejunum.Am J Physiol Gastrointest Liver Physiol. 2009 Apr;296(4):G833-9. doi: 10.1152/ajpgi.90610.2008. Epub 2009 Jan 29. Am J Physiol Gastrointest Liver Physiol. 2009. PMID: 19179622 Free PMC article.
References
-
- Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signaling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. - PubMed
-
- Levade T, Auge N, Veldman RJ, Cuvillier O, Negre-Salvayre A, Salvayre R. Sphingolipid mediators in cardiovascular cell biology and pathology. Circ Res. 2001;89:957–968. - PubMed
-
- Saba JD, Hla T. Point-counterpoint of sphingosine 1-phosphate metabolism. Circ Res. 2004;94:724–734. - PubMed
-
- Jin ZQ, Goetzl EJ, Karliner JS. Sphingosine kinase activation mediates ischemic preconditioning in murine heart. Circulation. 2004;110:1980–1989. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

