Distinct mechanisms of clathrin-independent endocytosis have unique sphingolipid requirements
- PMID: 16672382
- PMCID: PMC1552047
- DOI: 10.1091/mbc.e05-12-1101
Distinct mechanisms of clathrin-independent endocytosis have unique sphingolipid requirements
Abstract
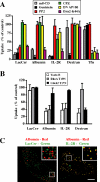
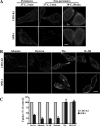
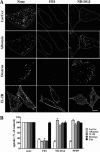
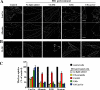
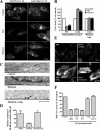
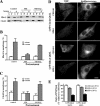
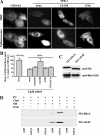
Sphingolipids (SLs) play important roles in membrane structure and cell function. Here, we examine the SL requirements of various endocytic mechanisms using a mutant cell line and pharmacological inhibitors to disrupt SL biosynthesis. First, we demonstrated that in Chinese hamster ovary cells we could distinguish three distinct mechanisms of clathrin-independent endocytosis (caveolar, RhoA, and Cdc42 dependent) which differed in cargo, sensitivity to pharmacological agents, and dominant negative proteins. General depletion of SLs inhibited endocytosis by each clathrin-independent mechanism, whereas clathrin-dependent uptake was unaffected. Depletion of glycosphingolipids (GSLs; a subgroup of SLs) selectively blocked caveolar endocytosis and decreased caveolin-1 and caveolae at the plasma membrane. Caveolar endocytosis and PM caveolae could be restored in GSL-depleted cells by acute addition of exogenous GSLs. Disruption of RhoA- and Cdc42-regulated endocytosis by SL depletion was shown to be related to decreased targeting of these Rho proteins to the plasma membrane and could be partially restored by exogenous sphingomyelin but not GSLs. Both the in vivo membrane targeting and in vitro binding to artificial lipid vesicles of RhoA and Cdc42 were shown to be dependent upon sphingomyelin. These results provide the first evidence that SLs are differentially required for distinct mechanisms of clathrin-independent endocytosis.
Figures
Similar articles
-
Co-regulation of caveolar and Cdc42-dependent fluid phase endocytosis by phosphocaveolin-1.J Biol Chem. 2010 May 14;285(20):15119-15125. doi: 10.1074/jbc.M109.069427. Epub 2010 Mar 12. J Biol Chem. 2010. PMID: 20228056 Free PMC article.
-
Membrane microdomains, caveolae, and caveolar endocytosis of sphingolipids.Mol Membr Biol. 2006 Jan-Feb;23(1):101-10. doi: 10.1080/09687860500460041. Mol Membr Biol. 2006. PMID: 16611585 Review.
-
Selective caveolin-1-dependent endocytosis of glycosphingolipids.Mol Biol Cell. 2003 Aug;14(8):3254-65. doi: 10.1091/mbc.e02-12-0809. Epub 2003 May 18. Mol Biol Cell. 2003. PMID: 12925761 Free PMC article.
-
Prominin-2 expression increases protrusions, decreases caveolae and inhibits Cdc42 dependent fluid phase endocytosis.Biochem Biophys Res Commun. 2013 May 10;434(3):466-72. doi: 10.1016/j.bbrc.2013.03.097. Epub 2013 Apr 10. Biochem Biophys Res Commun. 2013. PMID: 23583380 Free PMC article.
-
Caveosomes and endocytosis of lipid rafts.J Cell Sci. 2003 Dec 1;116(Pt 23):4707-14. doi: 10.1242/jcs.00840. J Cell Sci. 2003. PMID: 14600257 Review.
Cited by
-
Caspase-activated cell-penetrating peptides reveal temporal coupling between endosomal release and apoptosis in an RGC-5 cell model.Bioconjug Chem. 2012 Sep 19;23(9):1783-93. doi: 10.1021/bc300036z. Epub 2012 Aug 30. Bioconjug Chem. 2012. PMID: 22900707 Free PMC article.
-
Heat shock protein 90 mediates efficient antigen cross presentation through the scavenger receptor expressed by endothelial cells-I.J Immunol. 2010 Sep 1;185(5):2903-17. doi: 10.4049/jimmunol.0903635. Epub 2010 Aug 4. J Immunol. 2010. PMID: 20686127 Free PMC article.
-
Sphingomyelin is important for the cellular entry and intracellular localization of Helicobacter pylori VacA.Cell Microbiol. 2010 Oct;12(10):1517-33. doi: 10.1111/j.1462-5822.2010.01487.x. Cell Microbiol. 2010. PMID: 20545942 Free PMC article.
-
Anomalous surface distribution of glycosyl phosphatidyl inositol-anchored proteins in neurons lacking acid sphingomyelinase.Mol Biol Cell. 2008 Feb;19(2):509-22. doi: 10.1091/mbc.e07-05-0439. Epub 2007 Nov 21. Mol Biol Cell. 2008. PMID: 18032586 Free PMC article.
-
Mechanisms and functions of endocytosis in T cells.Cell Commun Signal. 2021 Sep 9;19(1):92. doi: 10.1186/s12964-021-00766-3. Cell Commun Signal. 2021. PMID: 34503523 Free PMC article. Review.
References
-
- Andrieu N., Salvayre R., Levade T. Comparative study of the metabolic pools of sphingomyelin and phosphatidylcholine sensitive to tumor necrosis factor. Eur. J. Biochem. 1996;236:738–745. - PubMed
-
- Bito R., Hino S., Baba A., Tanaka M., Watabe H., Kawabata H. Degradation of oxidative stress-induced denatured albumin in rat liver endothelial cells. Am. J. Physiol. 2005;289:C531–C542. - PubMed
-
- Brown D. A., London E. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 1998;14:111–136. - PubMed
-
- Chen C.-S., Rosenwald A. G., Pagano R. E. Ceramide as a modulator of endocytosis. J. Biol. Chem. 1995;270:13291–13297. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

