Distinct timing in the activity of cannabinoid-sensitive and cannabinoid-insensitive basket cells
- PMID: 16648849
- PMCID: PMC3509385
- DOI: 10.1038/nn1688
Distinct timing in the activity of cannabinoid-sensitive and cannabinoid-insensitive basket cells
Abstract
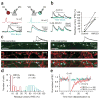
Cannabinoids are powerful modulators of inhibition, yet the precise spike timing of cannabinoid receptor (CB1R)-expressing inhibitory neurons in relation to other neurons in the circuit is poorly understood. Here we found that the spike timing of CB1R-expressing basket cells, a major target for cannabinoids in the rat hippocampus, was distinct from the other main group of basket cells, the CB1R-negative. Despite receiving the same afferent inputs, the synaptic and biophysical properties of the two cell types were tuned to detect different features of activity. CB1R-negative basket cells responded reliably and immediately to subtle and repetitive excitation. In contrast, CB1R-positive basket cells responded later and did not follow repetitive activity, but were better suited to integrate the consecutive excitation of independent afferents. This temporal separation in the activity of the two basket cell types generated distinct epochs of somatic inhibition that were differentially affected by endocannabinoids.
Conflict of interest statement
The authors declare that they have no competing financial interests.
Figures



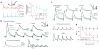

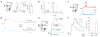
Similar articles
-
Interneurons targeting similar layers receive synaptic inputs with similar kinetics.Hippocampus. 2006;16(4):408-20. doi: 10.1002/hipo.20169. Hippocampus. 2006. PMID: 16435315
-
Cell type-specific long-term plasticity at glutamatergic synapses onto hippocampal interneurons expressing either parvalbumin or CB1 cannabinoid receptor.J Neurosci. 2010 Jan 27;30(4):1337-47. doi: 10.1523/JNEUROSCI.3481-09.2010. J Neurosci. 2010. PMID: 20107060 Free PMC article.
-
Novel cannabinoid-sensitive receptor mediates inhibition of glutamatergic synaptic transmission in the hippocampus.Neuroscience. 2001;106(1):1-4. doi: 10.1016/s0306-4522(01)00287-1. Neuroscience. 2001. PMID: 11564411
-
Distinct cannabinoid sensitive receptors regulate hippocampal excitation and inhibition.Chem Phys Lipids. 2002 Dec 31;121(1-2):73-82. doi: 10.1016/s0009-3084(02)00149-4. Chem Phys Lipids. 2002. PMID: 12505692 Review.
-
Endocannabinoids and cortical plasticity: CB1R as a possible regulator of the excitation/inhibition balance in health and disease.Eur J Neurosci. 2022 Feb;55(4):971-988. doi: 10.1111/ejn.15110. Epub 2021 Feb 6. Eur J Neurosci. 2022. PMID: 33427341 Review.
Cited by
-
Functional characteristics of parvalbumin- and cholecystokinin-expressing basket cells.J Physiol. 2012 Feb 15;590(4):669-81. doi: 10.1113/jphysiol.2011.226175. Epub 2012 Jan 16. J Physiol. 2012. PMID: 22250212 Free PMC article. Review.
-
Short-term plasticity regulates the excitation/inhibition ratio and the temporal window for spike integration in CA1 pyramidal cells.Eur J Neurosci. 2015 May;41(11):1402-15. doi: 10.1111/ejn.12898. Epub 2015 Apr 23. Eur J Neurosci. 2015. PMID: 25903384 Free PMC article.
-
Computational modeling of GABAA receptor-mediated paired-pulse inhibition in the dentate gyrus.J Comput Neurosci. 2010 Dec;29(3):509-19. doi: 10.1007/s10827-010-0214-y. Epub 2010 Feb 23. J Comput Neurosci. 2010. PMID: 20177763
-
NPAS4 recruits CCK basket cell synapses and enhances cannabinoid-sensitive inhibition in the mouse hippocampus.Elife. 2018 Jul 27;7:e35927. doi: 10.7554/eLife.35927. Elife. 2018. PMID: 30052197 Free PMC article.
-
Stringent specificity in the construction of a GABAergic presynaptic inhibitory circuit.Cell. 2009 Oct 2;139(1):161-74. doi: 10.1016/j.cell.2009.08.027. Cell. 2009. PMID: 19804761 Free PMC article.
References
-
- Andersen P, Eccles JC, Loyning Y. Recurrent inhibition in the hippocampus with identification of the inhibitory cell and its synapses. Nature. 1963;198:540–542. - PubMed
-
- Mann EO, Suckling JM, Hajos N, Greenfield SA, Paulsen O. Perisomatic feedback inhibition underlies cholinergically induced fast network oscillations in the rat hippocampus in vitro. Neuron. 2005;45:105–117. - PubMed
-
- Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature. 1995;378:75–78. - PubMed
-
- Miles R, Toth K, Gulyas AI, Hajos N, Freund TF. Differences between somatic and dendritic inhibition in the hippocampus. Neuron. 1996;16:815–823. - PubMed
-
- Pouille F, Scanziani M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science. 2001;293:1159–1163. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

