A role for synaptotagmin VII-regulated exocytosis of lysosomes in neurite outgrowth from primary sympathetic neurons
- PMID: 16641243
- PMCID: PMC6674075
- DOI: 10.1523/JNEUROSCI.0009-06.2006
A role for synaptotagmin VII-regulated exocytosis of lysosomes in neurite outgrowth from primary sympathetic neurons
Abstract
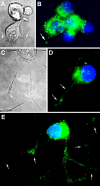
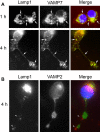
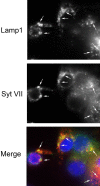
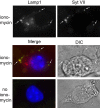
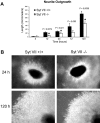
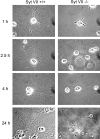
Neurite outgrowth is mediated by the exocytosis of intracellular vesicles at the tips of elongating neuronal processes. The lysosomal vesicle-associated soluble N-ethylmaleimide-sensitive factor attachment protein receptor tetanus neurotoxin insensitive vesicle-associated membrane protein (TI-VAMP)/VAMP7 was previously implicated in membrane fusion events mediating neurite outgrowth, but the participation of lysosomes in this exocytic process has remained unclear. Here, we show that VAMP7 and the lysosomal glycoprotein Lamp1 extensively colocalize in vesicles present throughout the soma and neurite outgrowths of primary sympathetic neurons. Synaptotagmin VII (Syt VII), a Ca(2+)-sensing synaptotagmin isoform previously shown to interact with VAMP7 during lysosomal exocytosis in fibroblasts, was detected on a subset of these lysosomal glycoprotein 1 (Lamp1)/VAMP7-positive neuronal vesicles. Ionophore-stimulated exocytosis triggered exposure of the luminal domains of both Lamp1 and Syt VII at overlapping sites on the neuronal surface, indicating that the Syt VII-containing lysosomal compartments fuse with the plasma membrane in response to [Ca2+]i elevation. To determine whether Syt VII was required for the exocytic events mediating neurite extension, we followed the development of superior cervical ganglion neurons explanted from Syt VII-deficient mice. The results revealed a marked defect in neurite outgrowth and arborization, suggesting that Ca(2+)-dependent, Syt VII-regulated exocytosis of late endosomes/lysosomes plays a role in the addition of new membrane to developing neurite extensions.
Figures
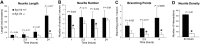
Similar articles
-
Injured astrocytes are repaired by Synaptotagmin XI-regulated lysosome exocytosis.Cell Death Differ. 2016 Apr;23(4):596-607. doi: 10.1038/cdd.2015.124. Epub 2015 Oct 9. Cell Death Differ. 2016. PMID: 26450452 Free PMC article.
-
Identification of SNAREs involved in synaptotagmin VII-regulated lysosomal exocytosis.J Biol Chem. 2004 May 7;279(19):20471-9. doi: 10.1074/jbc.M400798200. Epub 2004 Mar 1. J Biol Chem. 2004. PMID: 14993220
-
Synaptotagmin VII regulates Ca(2+)-dependent exocytosis of lysosomes in fibroblasts.J Cell Biol. 2000 Mar 20;148(6):1141-49. doi: 10.1083/jcb.148.6.1141. J Cell Biol. 2000. PMID: 10725327 Free PMC article.
-
Synaptotagmin regulates mast cell functions.Immunol Rev. 2001 Feb;179:25-34. doi: 10.1034/j.1600-065x.2001.790103.x. Immunol Rev. 2001. PMID: 11292024 Review.
-
There's more to life than neurotransmission: the regulation of exocytosis by synaptotagmin VII.Trends Cell Biol. 2005 Nov;15(11):626-31. doi: 10.1016/j.tcb.2005.09.001. Epub 2005 Sep 15. Trends Cell Biol. 2005. PMID: 16168654 Review.
Cited by
-
Repeated ER-endosome contacts promote endosome translocation and neurite outgrowth.Nature. 2015 Apr 9;520(7546):234-8. doi: 10.1038/nature14359. Nature. 2015. PMID: 25855459
-
Cathepsins in neuronal plasticity.Neural Regen Res. 2021 Jan;16(1):26-35. doi: 10.4103/1673-5374.286948. Neural Regen Res. 2021. PMID: 32788444 Free PMC article.
-
Regulation of lysosomal secretion by cortactin drives fibronectin deposition and cell motility.Bioarchitecture. 2011 Nov 1;1(6):257-260. doi: 10.4161/bioa.1.6.19197. Bioarchitecture. 2011. PMID: 22545176 Free PMC article.
-
Extracellular Vesicle-Mediated Cell⁻Cell Communication in the Nervous System: Focus on Neurological Diseases.Int J Mol Sci. 2019 Jan 20;20(2):434. doi: 10.3390/ijms20020434. Int J Mol Sci. 2019. PMID: 30669512 Free PMC article. Review.
-
Drosophila Rab2 controls endosome-lysosome fusion and LAMP delivery to late endosomes.Autophagy. 2018;14(9):1520-1542. doi: 10.1080/15548627.2018.1458170. Epub 2018 Aug 13. Autophagy. 2018. PMID: 29940804 Free PMC article.
References
-
- Alberts P, Rudge R, Hinners I, Muzerelle A, Martinez-Arca S, Irinopoulou T, Marthiens V, Tooze S, Rathjen F, Gaspar P, Galli T (2003). Cross talk between tetanus neurotoxin-insensitive vesicle-associated membrane protein-mediated transport and L1-mediated adhesion. Mol Biol Cell 14:4207–4220. - PMC - PubMed
-
- Andrews NW, Chakrabarti S (2005). There’s more to life than neurotransmission: the regulation of exocytosis by synaptotagmin VII. Trends Cell Biol 15:626–631. - PubMed
-
- Arantes RM, Lourenssen S, Machado CR, Blennerhassett MG (2000). Early damage of sympathetic neurons after co-culture with macrophages: a model of neuronal injury in vitro. NeuroReport 11:177–181. - PubMed
-
- Bradke F, Dotti CG (1997). Neuronal polarity: vectorial cytoplasmic flow precedes axon formation. Neuron 19:1175–1186. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
