Conversion to the amyotrophic lateral sclerosis phenotype is associated with intermolecular linked insoluble aggregates of SOD1 in mitochondria
- PMID: 16636275
- PMCID: PMC1447523
- DOI: 10.1073/pnas.0602046103
Conversion to the amyotrophic lateral sclerosis phenotype is associated with intermolecular linked insoluble aggregates of SOD1 in mitochondria
Abstract
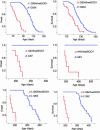
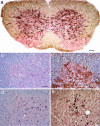
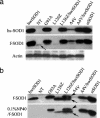
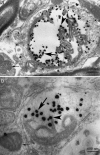
Twenty percent of the familial form of amyotrophic lateral sclerosis (ALS) is caused by mutations in the Cu, Zn-superoxide dismutase gene (SOD1) through the gain of a toxic function. The nature of this toxic function of mutant SOD1 has remained largely unknown. Here we show that WT SOD1 not only hastens onset of the ALS phenotype but can also convert an unaffected phenotype to an ALS phenotype in mutant SOD1 transgenic mouse models. Further analyses of the single- and double-transgenic mice revealed that conversion of mutant SOD1 from a soluble form to an aggregated and detergent-insoluble form was associated with development of the ALS phenotype in transgenic mice. Conversion of WT SOD1 from a soluble form to an aggregated and insoluble form also correlates with exacerbation of the disease or conversion to a disease phenotype in double-transgenic mice. This conversion, observed in the mitochondrial fraction of the spinal cord, involved formation of insoluble SOD1 dimers and multimers that are crosslinked through intermolecular disulfide bonds via oxidation of cysteine residues in SOD1. Our data thus show a molecular mechanism by which SOD1, an important protein in cellular defense against free radicals, is converted to aggregated and apparently ALS-associated toxic dimers and multimers by redox processes. These findings provide evidence of direct links among oxidation, protein aggregation, mitochondrial damage, and SOD1-mediated ALS, with possible applications to the aging process and other late-onset neurodegenerative disorders. Importantly, rational therapy based on these observations can now be developed and tested.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
Similar articles
-
Disulfide cross-linked protein represents a significant fraction of ALS-associated Cu, Zn-superoxide dismutase aggregates in spinal cords of model mice.Proc Natl Acad Sci U S A. 2006 May 2;103(18):7148-53. doi: 10.1073/pnas.0602048103. Epub 2006 Apr 24. Proc Natl Acad Sci U S A. 2006. PMID: 16636274 Free PMC article.
-
Human Cu/Zn superoxide dismutase (SOD1) overexpression in mice causes mitochondrial vacuolization, axonal degeneration, and premature motoneuron death and accelerates motoneuron disease in mice expressing a familial amyotrophic lateral sclerosis mutant SOD1.Neurobiol Dis. 2000 Dec;7(6 Pt B):623-43. doi: 10.1006/nbdi.2000.0299. Neurobiol Dis. 2000. PMID: 11114261
-
Disulfide bond mediates aggregation, toxicity, and ubiquitylation of familial amyotrophic lateral sclerosis-linked mutant SOD1.J Biol Chem. 2007 Sep 21;282(38):28087-95. doi: 10.1074/jbc.M704465200. Epub 2007 Jul 31. J Biol Chem. 2007. PMID: 17666395
-
Mitochondrial dysfunction in familial amyotrophic lateral sclerosis.J Bioenerg Biomembr. 2011 Dec;43(6):587-92. doi: 10.1007/s10863-011-9393-0. J Bioenerg Biomembr. 2011. PMID: 22072073 Review.
-
Aggregation of copper-zinc superoxide dismutase in familial and sporadic ALS.Antioxid Redox Signal. 2009 Jul;11(7):1603-14. doi: 10.1089/ars.2009.2536. Antioxid Redox Signal. 2009. PMID: 19271992 Free PMC article. Review.
Cited by
-
VDAC1-Based Peptides as Potential Modulators of VDAC1 Interactions with Its Partners and as a Therapeutic for Cancer, NASH, and Diabetes.Biomolecules. 2024 Sep 9;14(9):1139. doi: 10.3390/biom14091139. Biomolecules. 2024. PMID: 39334905 Free PMC article. Review.
-
Ebselen analogues delay disease onset and its course in fALS by on-target SOD-1 engagement.Sci Rep. 2024 May 27;14(1):12118. doi: 10.1038/s41598-024-62903-5. Sci Rep. 2024. PMID: 38802492 Free PMC article.
-
Amyotrophic Lateral Sclerosis-Associated Mutants of SOD1 Perturb mRNA Splicing through Aberrant Interactions with SRSF2.Anal Chem. 2024 Jun 11;96(23):9713-9720. doi: 10.1021/acs.analchem.4c01770. Epub 2024 May 25. Anal Chem. 2024. PMID: 38795036
-
Mitochondria: A Promising Convergent Target for the Treatment of Amyotrophic Lateral Sclerosis.Cells. 2024 Jan 29;13(3):248. doi: 10.3390/cells13030248. Cells. 2024. PMID: 38334639 Free PMC article. Review.
-
Pathophysiology of ion channels in amyotrophic lateral sclerosis.Mol Brain. 2023 Dec 15;16(1):82. doi: 10.1186/s13041-023-01070-6. Mol Brain. 2023. PMID: 38102715 Free PMC article. Review.
References
-
- Hughes J. T. Adv. Neurol. 1982;36:61–74. - PubMed
-
- Lacomblez L., Bensimon G., Leigh P. N., Guillet P., Meininger V. Lancet. 1996;347:1425–1431. - PubMed
-
- Rosen D. R., Siddique T., Patterson D., Figlewicz D. A., Sapp P., Hentati A., Donaldson D., Goto J., O'Regan J. P., Deng H.-X., et al. Nature. 1993;362:59–62. - PubMed
-
- Deng H.-X., Hentati A., Tainer J. A., Iqbal Z., Cayabyab A., Hung W. Y., Getzoff E. D., Hu P., Herzfeldt B., Roos R. P., et al. Science. 1993;261:1047–1051. - PubMed
-
- Gurney M. E., Pu H., Chiu A. Y., Dal Canto M. C., Polchow C. Y., Alexander D. D., Caliendo J., Hentati A., Kwon Y. W., Deng H.-X., et al. Science. 1994;264:1772–1775. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

