Toll-like receptor 2 signaling modulates the functions of CD4+ CD25+ regulatory T cells
- PMID: 16632602
- PMCID: PMC1444884
- DOI: 10.1073/pnas.0601554103
Toll-like receptor 2 signaling modulates the functions of CD4+ CD25+ regulatory T cells
Abstract
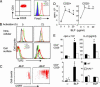
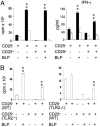
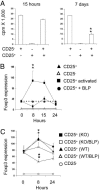
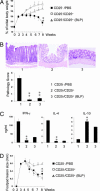
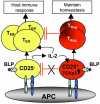
Toll-like receptors (TLRs) are primary sensors of both innate and adaptive immune systems and play a pivotal role in response against structurally conserved components of pathogens. Synthetic bacterial lipoprotein (BLP) Pam3Cys-SK4 is a TLR2 agonist that is capable of modulating T cell immune responses. We show here that BLP, together with anti-CD3 antibody [T cell receptor (TcR) activation], induced proliferation of both CD4+ CD25+ regulatory T cells (Tregs) and CD4+ CD25- (effector) T cells in the absence of antigen-presenting cells. The expanded Tregs showed a transient loss of suppressive activity. Moreover, BLP rendered effectors resistant to the suppression of Tregs by increasing IL-2 secretion. BLP also transiently suppressed the induction of Foxp3 (X-linked forkhead/winged helix transcription factor) mRNA in Tregs at the first 8-15 h after T cell receptor activation. Consistent with this observation, BLP-stimulated Tregs regained their inhibitory activity and prevented spontaneous colitis induced by effectors in severe combined immunodeficient mice. Our results demonstrate a previously unrecognized pathway by which TLR expressed on T cells may directly modulate the immune response. Thus, during an acute bacterial infection, BLP may rapidly increase the host's adaptive immunity by expanding effectors and also by attenuating the suppressive activity of Tregs. In the process, BLP also expands the Tregs, which recover their suppressive activity when the infection has subsided, in time to limit potential autoimmunity that might result from the overactivated effectors.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures

Similar articles
-
Toll-like receptor 2-mediated modulation of growth and functions of regulatory T cells by oral streptococci.Mol Oral Microbiol. 2013 Aug;28(4):267-80. doi: 10.1111/omi.12023. Epub 2013 Feb 18. Mol Oral Microbiol. 2013. PMID: 23413817
-
Stimulation of α7 nicotinic acetylcholine receptor by nicotine increases suppressive capacity of naturally occurring CD4+CD25+ regulatory T cells in mice in vitro.J Pharmacol Exp Ther. 2010 Dec;335(3):553-61. doi: 10.1124/jpet.110.169961. Epub 2010 Sep 15. J Pharmacol Exp Ther. 2010. PMID: 20843956
-
Toll-like receptor 2 controls expansion and function of regulatory T cells.J Clin Invest. 2006 Feb;116(2):485-94. doi: 10.1172/JCI25439. Epub 2006 Jan 19. J Clin Invest. 2006. PMID: 16424940 Free PMC article.
-
Dendritic cells expand antigen-specific Foxp3+ CD25+ CD4+ regulatory T cells including suppressors of alloreactivity.Immunol Rev. 2006 Aug;212:314-29. doi: 10.1111/j.0105-2896.2006.00422.x. Immunol Rev. 2006. PMID: 16903923 Review.
-
The development and function of regulatory T cells.Cell Mol Life Sci. 2009 Aug;66(16):2603-22. doi: 10.1007/s00018-009-0026-2. Epub 2009 Apr 24. Cell Mol Life Sci. 2009. PMID: 19390784 Free PMC article. Review.
Cited by
-
Toll-like receptor 4 activation in cancer progression and therapy.Clin Dev Immunol. 2011;2011:609579. doi: 10.1155/2011/609579. Epub 2011 Nov 3. Clin Dev Immunol. 2011. PMID: 22110526 Free PMC article. Review.
-
Toll-Like Receptor-Based Strategies for Cancer Immunotherapy.J Immunol Res. 2021 May 22;2021:9912188. doi: 10.1155/2021/9912188. eCollection 2021. J Immunol Res. 2021. PMID: 34124272 Free PMC article. Review.
-
Resolution of inflammation: state of the art, definitions and terms.FASEB J. 2007 Feb;21(2):325-32. doi: 10.1096/fj.06-7227rev. FASEB J. 2007. PMID: 17267386 Free PMC article. Review.
-
Linkage between Toll-like receptor (TLR) 2 promotor and intron polymorphisms: functional effects and relevance to sarcoidosis.Clin Exp Immunol. 2007 Sep;149(3):453-62. doi: 10.1111/j.1365-2249.2007.03428.x. Epub 2007 Jun 12. Clin Exp Immunol. 2007. PMID: 17565608 Free PMC article.
-
Heat shock proteins induce T cell regulation of chronic inflammation.Ann Rheum Dis. 2006 Nov;65 Suppl 3(Suppl 3):iii65-8. doi: 10.1136/ard.2006.058495. Ann Rheum Dis. 2006. PMID: 17038477 Free PMC article. Review.
References
-
- Chen Y., Kuchroo V. K., Inobe J., Hafler D. A., Weiner H. L. Science. 1994;265:1237–1240. - PubMed
-
- Sakaguchi S., Sakaguchi N., Asano M., Itoh M., Toda M. J. Immunol. 1995;155:1151–1164. - PubMed
-
- Groux H., O’Garra A., Bigler M., Rouleau M., Antonenko S., de Vries J. E., Roncarolo M. G. Nature. 1997;389:737–742. - PubMed
-
- Medzhitov R., Preston-Hurlburt P., Janeway C. A., Jr. Nature. 1997;388:394–397. - PubMed
-
- Janeway C. A., Jr., Medzhitov R. Annu. Rev. Immunol. 2002;20:197–216. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

