Activated microglia contribute to the maintenance of chronic pain after spinal cord injury
- PMID: 16624951
- PMCID: PMC6674010
- DOI: 10.1523/JNEUROSCI.0003-06.2006
Activated microglia contribute to the maintenance of chronic pain after spinal cord injury
Abstract
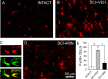
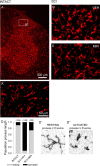
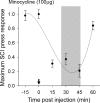
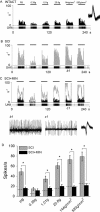
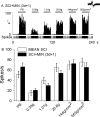
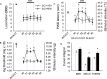
Traumatic spinal cord injury (SCI) results not only in motor impairment but also in chronic central pain, which can be refractory to conventional treatment approaches. It has been shown recently that in models of peripheral nerve injury, spinal cord microglia can become activated and contribute to development of pain. Considering their role in pain after peripheral injury, and because microglia are known to become activated after SCI, we tested the hypothesis that activated microglia contribute to chronic pain after SCI. In this study, adult male Sprague Dawley rats underwent T9 spinal cord contusion injury. Four weeks after injury, when lumbar dorsal horn multireceptive neurons became hyperresponsive and when behavioral nociceptive thresholds were decreased to both mechanical and thermal stimuli, intrathecal infusions of the microglial inhibitor minocycline were initiated. Electrophysiological experiments showed that minocycline rapidly attenuated hyperresponsiveness of lumbar dorsal horn neurons. Behavioral data showed that minocycline restored nociceptive thresholds, at which time spinal microglial cells assumed a quiescent morphological phenotype. Levels of phosphorylated-p38 were decreased in SCI animals receiving minocycline. Cessation of delivery of minocycline resulted in an immediate return of pain-related phenomena. These results suggest an important role for activated microglia in the maintenance of chronic central below-level pain after SCI and support the newly emerging role of non-neuronal immune cells as a contributing factor in post-SCI pain.
Figures


Similar articles
-
Extracellular signal-regulated kinase-regulated microglia-neuron signaling by prostaglandin E2 contributes to pain after spinal cord injury.J Neurosci. 2007 Feb 28;27(9):2357-68. doi: 10.1523/JNEUROSCI.0138-07.2007. J Neurosci. 2007. PMID: 17329433 Free PMC article.
-
IL-6/JAK2/STAT3 axis mediates neuropathic pain by regulating astrocyte and microglia activation after spinal cord injury.Exp Neurol. 2023 Dec;370:114576. doi: 10.1016/j.expneurol.2023.114576. Epub 2023 Oct 18. Exp Neurol. 2023. PMID: 37863306
-
Early microglial inhibition preemptively mitigates chronic pain development after experimental spinal cord injury.J Rehabil Res Dev. 2009;46(1):123-33. J Rehabil Res Dev. 2009. PMID: 19533525
-
Involvement of microglia in chronic neuropathic pain associated with spinal cord injury - a systematic review.Rev Neurosci. 2023 Jul 26;34(8):933-950. doi: 10.1515/revneuro-2023-0031. Print 2023 Dec 15. Rev Neurosci. 2023. PMID: 37490300 Review.
-
Neuroplasticity related to chronic pain and its modulation by microglia.Inflamm Regen. 2022 May 3;42(1):15. doi: 10.1186/s41232-022-00199-6. Inflamm Regen. 2022. PMID: 35501933 Free PMC article. Review.
Cited by
-
Mechanisms and Therapeutic Prospects of Microglia-Astrocyte Interactions in Neuropathic Pain Following Spinal Cord Injury.Mol Neurobiol. 2024 Oct 29. doi: 10.1007/s12035-024-04562-1. Online ahead of print. Mol Neurobiol. 2024. PMID: 39470872 Review.
-
Reciprocal modulation between microglia and astrocyte in reactive gliosis following the CNS injury.Mol Neurobiol. 2013 Dec;48(3):690-701. doi: 10.1007/s12035-013-8460-4. Epub 2013 Apr 24. Mol Neurobiol. 2013. PMID: 23613214 Free PMC article. Review.
-
Inhibition of astrocyte hemichannel improves recovery from spinal cord injury.JCI Insight. 2021 Mar 8;6(5):e134611. doi: 10.1172/jci.insight.134611. JCI Insight. 2021. PMID: 33682795 Free PMC article.
-
Astrocytic CX43 hemichannels and gap junctions play a crucial role in development of chronic neuropathic pain following spinal cord injury.Glia. 2012 Nov;60(11):1660-70. doi: 10.1002/glia.22384. Epub 2012 Aug 1. Glia. 2012. PMID: 22951907 Free PMC article.
-
Extracellular signal-regulated kinase-regulated microglia-neuron signaling by prostaglandin E2 contributes to pain after spinal cord injury.J Neurosci. 2007 Feb 28;27(9):2357-68. doi: 10.1523/JNEUROSCI.0138-07.2007. J Neurosci. 2007. PMID: 17329433 Free PMC article.
References
-
- Afrah AW, Fiska A, Gjerstad J, Gustafsson H, Tjolsen A, Olgart L, Stiller CO, Hole K, Brodin E (2002). Spinal substance P release in vivo during the induction of long-term potentiation in dorsal horn neurons. Pain 96:49–55. - PubMed
-
- Attal N, Guirimand F, Brasseur L, Gaude V, Chauvin M, Bouhassira D (2002). Effects of IV morphine in central pain: a randomized placebo-controlled study. Neurology 58:554–563. - PubMed
-
- Basso DM, Beattie MS, Bresnahan JC (1995). A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 12:1–21. - PubMed
-
- Cardenas DD, Warms CA, Turner JA, Marshall H, Brooke MM, Loeser JD (2002). Efficacy of amitriptyline for relief of pain in spinal cord injury: results of a randomized controlled trial. Pain 96:365–373. - PubMed
-
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL (1994). Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53:55–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
