Dynamics of multiple symbiont density regulation during host development: tsetse fly and its microbial flora
- PMID: 16618673
- PMCID: PMC1560226
- DOI: 10.1098/rspb.2005.3399
Dynamics of multiple symbiont density regulation during host development: tsetse fly and its microbial flora
Abstract
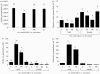
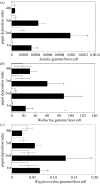
Symbiotic associations often enhance hosts' physiological capabilities, allowing them to expand into restricted terrains, thus leading to biological diversification. Stable maintenance of partners is essential for the overall biological system to succeed. The viviparous tsetse fly (Diptera: Glossinidae) offers an exceptional system to examine factors that influence the maintenance of multiple symbiotic organisms within a single eukaryotic host. This insect harbours three different symbionts representing diverse associations, coevolutionary histories and transmission modes. The enterics, obligate mutualist Wigglesworthia and beneficial Sodalis, are maternally transmitted to the intrauterine larvae, while parasitic Wolbachia infects the developing oocyte. In this study, the population dynamics of these three symbionts were examined through host development and during potentially disruptive events, including host immune challenge, the presence of third parties (such as African trypanosomes) and environmental perturbations (such as fluctuating humidity levels). While mutualistic partners exhibited well-regulated density profiles over different host developmental stages, parasitic Wolbachia infections varied in individual hosts. Host immune status and the presence of trypanosome infections did not impact the steady-state density levels observed for mutualistic microbes in either sex, while these factors resulted in an increase in Wolbachia density in males. Interestingly, perturbation of the maternal environment resulted in the deposition of progeny harbouring greater overall symbiont loads. The regulation of symbiont density, arising from coadaptive processes, may be an important mechanism driving inter-specific relations to ensure their competitive survival and to promote specialization of beneficial associations.
Figures


Similar articles
-
Wolbachia, Sodalis and trypanosome co-infections in natural populations of Glossina austeni and Glossina pallidipes.Parasit Vectors. 2013 Aug 8;6(1):232. doi: 10.1186/1756-3305-6-232. Parasit Vectors. 2013. PMID: 23924682 Free PMC article.
-
Bacterial Symbionts of Tsetse Flies: Relationships and Functional Interactions Between Tsetse Flies and Their Symbionts.Results Probl Cell Differ. 2020;69:497-536. doi: 10.1007/978-3-030-51849-3_19. Results Probl Cell Differ. 2020. PMID: 33263885 Review.
-
Prevalence of symbionts and trypanosome infections in tsetse flies of two villages of the "Faro and Déo" division of the Adamawa region of Cameroon.BMC Microbiol. 2018 Nov 23;18(Suppl 1):159. doi: 10.1186/s12866-018-1286-5. BMC Microbiol. 2018. PMID: 30470177 Free PMC article.
-
The obligate mutualist Wigglesworthia glossinidia influences reproduction, digestion, and immunity processes of its host, the tsetse fly.Appl Environ Microbiol. 2008 Oct;74(19):5965-74. doi: 10.1128/AEM.00741-08. Epub 2008 Aug 8. Appl Environ Microbiol. 2008. PMID: 18689507 Free PMC article.
-
Interactions among multiple genomes: tsetse, its symbionts and trypanosomes.Insect Biochem Mol Biol. 2005 Jul;35(7):691-8. doi: 10.1016/j.ibmb.2005.02.012. Epub 2005 Mar 28. Insect Biochem Mol Biol. 2005. PMID: 15894186 Review.
Cited by
-
An IMD-like pathway mediates both endosymbiont control and host immunity in the cereal weevil Sitophilus spp.Microbiome. 2018 Jan 8;6(1):6. doi: 10.1186/s40168-017-0397-9. Microbiome. 2018. PMID: 29310713 Free PMC article.
-
Interactions between mutualist Wigglesworthia and tsetse peptidoglycan recognition protein (PGRP-LB) influence trypanosome transmission.Proc Natl Acad Sci U S A. 2009 Jul 21;106(29):12133-8. doi: 10.1073/pnas.0901226106. Epub 2009 Jul 8. Proc Natl Acad Sci U S A. 2009. PMID: 19587241 Free PMC article.
-
The gut bacterial communities associated with lab-raised and field-collected ants of Camponotus fragilis (Formicidae: Formicinae).Curr Microbiol. 2014 Sep;69(3):292-302. doi: 10.1007/s00284-014-0586-8. Epub 2014 Apr 20. Curr Microbiol. 2014. PMID: 24748441
-
Intraspecific variation in immune gene expression and heritable symbiont density.PLoS Pathog. 2021 Apr 26;17(4):e1009552. doi: 10.1371/journal.ppat.1009552. eCollection 2021 Apr. PLoS Pathog. 2021. PMID: 33901257 Free PMC article.
-
Infection with the secondary tsetse-endosymbiont Sodalis glossinidius (Enterobacteriales: Enterobacteriaceae) influences parasitism in Glossina pallidipes (Diptera: Glossinidae).J Insect Sci. 2014 Jan 1;14:272. doi: 10.1093/jisesa/ieu134. Print 2014. J Insect Sci. 2014. PMID: 25527583 Free PMC article.
References
-
- Akman L, Yamashita A, Watanabe H, Oshima K, Shiba T, Hattori M, Aksoy S. Genome sequence of the endocellular obligate symbiont of tsetse, Wigglesworthia glossinidia. Nat. Genet. 2002;32:402–407. 10.1038/ng986 - DOI - PubMed
-
- Aksoy S. Molecular analysis of the endosymbionts of tsetse flies: 16S rDNA locus and over-expression of a chaperonin. Insect Mol. Biol. 1995;4:23–29. - PubMed
-
- Bandi C, Dunn A.M, Hurst G.D, Rigaud T. Inherited microorganisms, sex-specific virulence and reproductive parasites. Trends Parasitol. 2001;17:88–94. 10.1016/S1471-4922(00)01812-2 - DOI - PubMed
-
- Boulanger N, Brun R, Ehret-Sabatier L, Kunz C, Bulet P. Immunopeptides in the defense reactions of Glossina morsitans to bacterial and Trypanosoma brucei brucei infections. Insect Biochem. Mol. Biol. 2002;32:369–375. 10.1016/S0965-1748(02)00029-2 - DOI - PubMed
-
- Brun R, Jenni L, Tanner M, Schonenberger M, Schell K.F. Cultivation of vertebrate infective forms derived from metacyclic forms of pleomorphic Trypanosoma brucei stocks. Acta Trop. 1979;36:387–390. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

