Single-cell analysis of normal and FOXP3-mutant human T cells: FOXP3 expression without regulatory T cell development
- PMID: 16617117
- PMCID: PMC1458937
- DOI: 10.1073/pnas.0509484103
Single-cell analysis of normal and FOXP3-mutant human T cells: FOXP3 expression without regulatory T cell development
Erratum in
- Proc Natl Acad Sci U S A. 2006 Jun 13;103(24):9373
Abstract
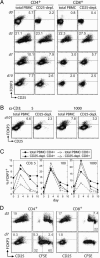
Forkhead winged-helix transcription factor Foxp3 serves as the dedicated mediator of the genetic program governing CD25+CD4+ regulatory T cell (T(R)) development and function in mice. In humans, its role in mediating T(R) development has been controversial. Furthermore, the fate of T(R) precursors in FOXP3 deficiency has yet to be described. Making use of flow cytometric detection of human FOXP3, we have addressed the relationship between FOXP3 expression and human T(R) development. Unlike murine Foxp3- T cells, a small subset of human CD4+ and CD8+ T cells transiently up-regulated FOXP3 upon in vitro stimulation. Induced FOXP3, however, did not alter cell-surface phenotype or suppress T helper 1 cytokine expression. Furthermore, only ex vivo FOXP3+ T(R) cells persisted after prolonged culture, suggesting that induced FOXP3 did not activate a T(r) developmental program in a significant number of cells. FOXP3 flow cytometry was also used to further characterize several patients exhibiting symptoms of immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) with or without FOXP3 mutations. Most patients lacked FOXP3-expressing cells, further solidifying the association between FOXP3 deficiency and immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome. Interestingly, one patient bearing a FOXP3 mutation enabling expression of stable FOXP3(mut) protein exhibited FOXP3(mut)-expressing cells among a subset of highly activated CD4+ T cells. This observation raises the possibility that the severe autoimmunity in FOXP3 deficiency can be attributed, in part, to aggressive T helper cells that have developed from T(R) precursors.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures


Similar articles
-
Identification of FOXP3-negative regulatory T-like (CD4(+)CD25(+)CD127(low)) cells in patients with immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome.Clin Immunol. 2011 Oct;141(1):111-20. doi: 10.1016/j.clim.2011.06.006. Epub 2011 Jul 12. Clin Immunol. 2011. PMID: 21802372
-
Defective regulatory and effector T cell functions in patients with FOXP3 mutations.J Clin Invest. 2006 Jun;116(6):1713-22. doi: 10.1172/JCI25112. J Clin Invest. 2006. PMID: 16741580 Free PMC article.
-
Functional type 1 regulatory T cells develop regardless of FOXP3 mutations in patients with IPEX syndrome.Eur J Immunol. 2011 Apr;41(4):1120-31. doi: 10.1002/eji.201040909. Epub 2011 Mar 14. Eur J Immunol. 2011. PMID: 21400500 Free PMC article.
-
Epigenetic mechanisms of regulation of Foxp3 expression.Blood. 2009 Oct 29;114(18):3727-35. doi: 10.1182/blood-2009-05-219584. Epub 2009 Jul 29. Blood. 2009. PMID: 19641188 Free PMC article. Review.
-
Immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) and IPEX-related disorders: an evolving web of heritable autoimmune diseases.Curr Opin Pediatr. 2013 Dec;25(6):708-14. doi: 10.1097/MOP.0000000000000029. Curr Opin Pediatr. 2013. PMID: 24240290 Free PMC article. Review.
Cited by
-
IL-21 promotes the expansion of CD27+ CD28+ tumor infiltrating lymphocytes with high cytotoxic potential and low collateral expansion of regulatory T cells.J Transl Med. 2013 Feb 12;11:37. doi: 10.1186/1479-5876-11-37. J Transl Med. 2013. PMID: 23402380 Free PMC article.
-
Boosting regulatory T cell function by CD4 stimulation enters the clinic.Front Immunol. 2012 Jun 18;3:164. doi: 10.3389/fimmu.2012.00164. eCollection 2012. Front Immunol. 2012. PMID: 22719741 Free PMC article.
-
Modulation of Suppressive Activity and Proliferation of Human Regulatory T Cells by Splice-Switching Oligonucleotides Targeting FoxP3 Pre-mRNA.Cells. 2023 Dec 29;13(1):77. doi: 10.3390/cells13010077. Cells. 2023. PMID: 38201281 Free PMC article.
-
FOXP3 expression diversifies the metabolic capacity and enhances the efficacy of CD8 T cells in adoptive immunotherapy of melanoma.Mol Ther. 2023 Jan 4;31(1):48-65. doi: 10.1016/j.ymthe.2022.08.017. Epub 2022 Aug 31. Mol Ther. 2023. PMID: 36045586 Free PMC article.
-
Epigenetic biomarkers of T-cells in human glioma.Epigenetics. 2012 Dec 1;7(12):1391-402. doi: 10.4161/epi.22675. Epub 2012 Oct 29. Epigenetics. 2012. PMID: 23108258 Free PMC article.
References
-
- Hori S., Nomura T., Sakaguchi S. Science. 2003;299:1057–1061. - PubMed
-
- Khattri R., Cox T., Yasayko S. A., Ramsdell F. Nat. Immunol. 2003;4:337–342. - PubMed
-
- Fontenot J. D., Gavin M. A., Rudensky A. Y. Nat. Immunol. 2003;4:330–336. - PubMed
-
- Baecher-Allan C., Viglietta V., Hafler D. A. Semin. Immunol. 2004;16:89–98. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

