Tangential neuronal migration controls axon guidance: a role for neuregulin-1 in thalamocortical axon navigation
- PMID: 16615895
- PMCID: PMC2365888
- DOI: 10.1016/j.cell.2006.01.042
Tangential neuronal migration controls axon guidance: a role for neuregulin-1 in thalamocortical axon navigation
Abstract
Neuronal migration and axon guidance constitute fundamental processes in brain development that are generally studied independently. Although both share common mechanisms of cell biology and biochemistry, little is known about their coordinated integration in the formation of neural circuits. Here we show that the development of the thalamocortical projection, one of the most prominent tracts in the mammalian brain, depends on the early tangential migration of a population of neurons derived from the ventral telencephalon. This tangential migration contributes to the establishment of a permissive corridor that is essential for thalamocortical axon pathfinding. Our results also demonstrate that in this process two different products of the Neuregulin-1 gene, CRD-NRG1 and Ig-NRG1, mediate the guidance of thalamocortical axons. These results show that neuronal tangential migration constitutes a novel mechanism to control the timely arrangement of guidance cues required for axonal tract formation in the mammalian brain.
Figures
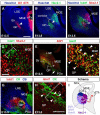
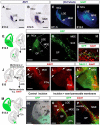
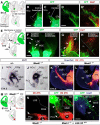
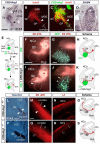
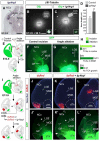
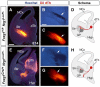
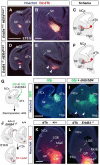
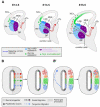
Comment in
-
Building bridges to the cortex.Cell. 2006 Apr 7;125(1):24-7. doi: 10.1016/j.cell.2006.03.021. Cell. 2006. PMID: 16615886
Similar articles
-
Building bridges to the cortex.Cell. 2006 Apr 7;125(1):24-7. doi: 10.1016/j.cell.2006.03.021. Cell. 2006. PMID: 16615886
-
Rac1 controls the formation of midline commissures and the competency of tangential migration in ventral telencephalic neurons.J Neurosci. 2007 Apr 4;27(14):3884-93. doi: 10.1523/JNEUROSCI.3509-06.2007. J Neurosci. 2007. PMID: 17409253 Free PMC article.
-
Structural Similarities between Neuregulin 1-3 Isoforms Determine Their Subcellular Distribution and Signaling Mode in Central Neurons.J Neurosci. 2017 May 24;37(21):5232-5249. doi: 10.1523/JNEUROSCI.2630-16.2017. Epub 2017 Apr 21. J Neurosci. 2017. PMID: 28432142 Free PMC article.
-
Inputs from the thalamocortical system on axon pathfinding mechanisms.Curr Opin Neurobiol. 2014 Aug;27:143-50. doi: 10.1016/j.conb.2014.03.013. Epub 2014 Apr 17. Curr Opin Neurobiol. 2014. PMID: 24742382 Review.
-
Mechanisms controlling the guidance of thalamocortical axons through the embryonic forebrain.Eur J Neurosci. 2012 May;35(10):1573-85. doi: 10.1111/j.1460-9568.2012.08119.x. Eur J Neurosci. 2012. PMID: 22607003 Free PMC article. Review.
Cited by
-
Development of the corticothalamic projections.Front Neurosci. 2012 May 4;6:53. doi: 10.3389/fnins.2012.00053. eCollection 2012. Front Neurosci. 2012. PMID: 22586359 Free PMC article.
-
Subrepellent doses of Slit1 promote Netrin-1 chemotactic responses in subsets of axons.Neural Dev. 2015 Mar 20;10:5. doi: 10.1186/s13064-015-0036-8. Neural Dev. 2015. PMID: 25888985 Free PMC article.
-
The genetics of brain wiring: from molecule to mind.PLoS Biol. 2007 Apr;5(4):e113. doi: 10.1371/journal.pbio.0050113. PLoS Biol. 2007. PMID: 17439300 Free PMC article.
-
Integrative mechanisms of oriented neuronal migration in the developing brain.Annu Rev Cell Dev Biol. 2013;29:299-353. doi: 10.1146/annurev-cellbio-101512-122400. Epub 2013 Aug 7. Annu Rev Cell Dev Biol. 2013. PMID: 23937349 Free PMC article. Review.
-
Mechanisms and molecules of neuronal wiring: a primer.Cold Spring Harb Perspect Biol. 2011 Jun 1;3(6):a001727. doi: 10.1101/cshperspect.a001727. Cold Spring Harb Perspect Biol. 2011. PMID: 21123392 Free PMC article. Review.
References
-
- Agmon A, Connors BW. Thalamocortical responses of mouse somatosensory (barrel) cortex in vitro. Neuroscience. 1991;41:365–379. - PubMed
-
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JLR. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997;278:474–476. - PubMed
-
- Auladell C, Pérez-Sust P, Supèr H, Soriano E. The early development of thalamocortical and corticothalamic projections in the mouse. Anat. Embryol. (Berl.) 2000;201:169–179. - PubMed
-
- Bagri A, Marín O, Plump AS, Mak J, Pleasure SJ, Rubenstein JL, Tessier-Lavigne M. Slit proteins prevent midline crossing and determine the dorsoventral position of major axonal pathways in the mammalian forebrain. Neuron. 2002;33:233–248. - PubMed
-
- Benson DL, Schnapp LM, Shapiro L, Huntley GW. Making memories stick: cell-adhesion molecules in synaptic plasticity. Trends Cell Biol. 2000;10:473–482. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

