Aging-dependent alterations in synaptic plasticity and memory in mice that overexpress extracellular superoxide dismutase
- PMID: 16611809
- PMCID: PMC6673899
- DOI: 10.1523/JNEUROSCI.5566-05.2006
Aging-dependent alterations in synaptic plasticity and memory in mice that overexpress extracellular superoxide dismutase
Abstract
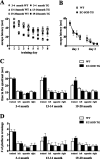
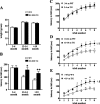
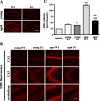
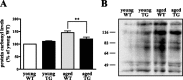
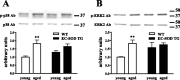
Oxidative damage caused by reactive oxygen species (ROS) has been proposed to be critically involved in several pathological manifestations of aging, including cognitive dysfunction. ROS, including superoxide, are generally considered as neurotoxic molecules whose effects can be alleviated by antioxidant enzymes. However, ROS also are known to be necessary components of the signal transduction cascades underlying normal synaptic plasticity. Therefore, we reasoned that the role that ROS and antioxidant enzymes play in modulating neuronal processes varies over the lifespan of an animal. We examined hippocampal long-term potentiation (LTP) and memory-related behavioral performance in transgenic mice overexpressing extracellular superoxide dismutase (EC-SOD) and their wild-type littermates at different ages. We found that aged EC-SOD transgenic mice exhibited enhanced hippocampal LTP, better cerebellum-dependent motor learning, and better hippocampus-dependent spatial learning compared with their wild-type littermates. We also found that EC-SOD overexpression impaired contextual learning, but the impairment was decreased in the aged transgenic mice. At the molecular level, aged EC-SOD transgenic mice had lower superoxide levels, a decrease in protein carbonyl levels, and a decrease in p38 and extracellular signal-regulated kinase 2 phosphorylation compared with aged wild-type mice. Our findings suggest that elevated levels of superoxide contribute to aging-related impairments in hippocampal LTP and memory, and that these impairments can be alleviated by overexpression of EC-SOD. We conclude that there is an age-dependent alteration in the role of superoxide in modulating synaptic plasticity and learning and memory.
Figures



Similar articles
-
Impairment of long-term potentiation and associative memory in mice that overexpress extracellular superoxide dismutase.J Neurosci. 2000 Oct 15;20(20):7631-9. doi: 10.1523/JNEUROSCI.20-20-07631.2000. J Neurosci. 2000. PMID: 11027223 Free PMC article.
-
Hippocampal long-term potentiation, memory, and longevity in mice that overexpress mitochondrial superoxide dismutase.Neurobiol Learn Mem. 2007 Mar;87(3):372-84. doi: 10.1016/j.nlm.2006.10.003. Epub 2006 Nov 28. Neurobiol Learn Mem. 2007. PMID: 17129739 Free PMC article.
-
Reactive oxygen species and synaptic plasticity in the aging hippocampus.Ageing Res Rev. 2004 Nov;3(4):431-43. doi: 10.1016/j.arr.2004.05.002. Ageing Res Rev. 2004. PMID: 15541710 Review.
-
Superoxide dismutase and hippocampal function: age and isozyme matter.Antioxid Redox Signal. 2007 Feb;9(2):201-10. doi: 10.1089/ars.2007.9.201. Antioxid Redox Signal. 2007. PMID: 17115934
-
Hippocampal memory and plasticity in superoxide dismutase mutant mice.Physiol Behav. 2002 Dec;77(4-5):601-5. doi: 10.1016/s0031-9384(02)00900-9. Physiol Behav. 2002. PMID: 12527006 Review.
Cited by
-
Insights into CNS ageing from animal models of senescence.Nat Rev Neurosci. 2012 May 10;13(6):435-45. doi: 10.1038/nrn3230. Nat Rev Neurosci. 2012. PMID: 22595787 Review.
-
A new K+channel-independent mechanism is involved in the antioxidant effect of XE-991 in an in vitro model of glucose metabolism impairment: implications for Alzheimer's disease.Cell Death Discov. 2022 Sep 20;8(1):391. doi: 10.1038/s41420-022-01187-y. Cell Death Discov. 2022. PMID: 36127342 Free PMC article.
-
Glucagon-like peptide-1 cleavage product GLP-1(9-36) amide rescues synaptic plasticity and memory deficits in Alzheimer's disease model mice.J Neurosci. 2012 Oct 3;32(40):13701-8. doi: 10.1523/JNEUROSCI.2107-12.2012. J Neurosci. 2012. PMID: 23035082 Free PMC article.
-
NOX activity is increased in mild cognitive impairment.Antioxid Redox Signal. 2010 Jun 15;12(12):1371-82. doi: 10.1089/ars.2009.2823. Antioxid Redox Signal. 2010. PMID: 19929442 Free PMC article.
-
Neurobiology of vascular dementia.J Aging Res. 2011;2011:401604. doi: 10.4061/2011/401604. Epub 2011 Aug 17. J Aging Res. 2011. PMID: 21876809 Free PMC article.
References
-
- Esposito F, Ammendola R, Faraonio R, Russo T, Cimino F (2004). Redox control of signal transduction, gene expression and cellular senescence. Neurochem Res 29:617–628. - PubMed
-
- Gahtan E, Auerbach JM, Groner Y, Segal M (1998). Reversible impairment of long-term potentiation in transgenic Cu/Zn-SOD mice. Eur J Neurosci 10:538–544. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

