Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion
- PMID: 16606668
- PMCID: PMC2118283
- DOI: 10.1084/jem.20051615
Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion
Abstract
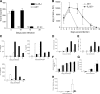
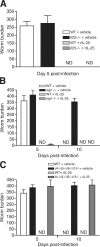
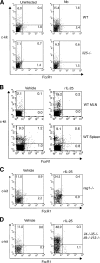
Type 2 immunity, which involves coordinated regulation of innate and adaptive immune responses, can protect against helminth parasite infection, but may lead to allergy and asthma after inappropriate activation. We demonstrate that il25(-/-) mice display inefficient Nippostrongylus brasiliensis expulsion and delayed cytokine production by T helper 2 cells. We further establish a key role for interleukin (IL)-25 in regulating a novel population of IL-4-, IL-5-, IL-13-producing non-B/non-T (NBNT), c-kit+, FcepsilonR1- cells during helminth infection. A deficit in this population in il25(-/-) mice correlates with inefficient N. brasiliensis expulsion. In contrast, administration of recombinant IL-25 in vivo induces the appearance of NBNT, c-kit+, FcepsilonR1- cells and leads to rapid worm expulsion that is T and B cell independent, but type 2 cytokine dependent. We demonstrate that these IL-25-regulated cells appear rapidly in the draining lymph nodes, implicating them as a source of type 2 cytokines during initiation of worm expulsion.
Figures


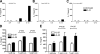
Comment in
-
Helper T cell differentiation enters a new era: le roi est mort; vive le roi!J Exp Med. 2006 Apr 17;203(4):809-12. doi: 10.1084/jem.20060522. Epub 2006 Apr 10. J Exp Med. 2006. PMID: 16606679 Free PMC article. Review.
Similar articles
-
Nippostrongylus brasiliensis: cytokine responses and nematode expulsion in normal and IL-4-deficient mice.Exp Parasitol. 1996 Oct;84(1):65-73. doi: 10.1006/expr.1996.0090. Exp Parasitol. 1996. PMID: 8888733
-
Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity.Nature. 2010 Apr 29;464(7293):1367-70. doi: 10.1038/nature08900. Epub 2010 Mar 3. Nature. 2010. PMID: 20200518 Free PMC article.
-
Basophil-mediated protection against gastrointestinal helminths requires IgE-induced cytokine secretion.Proc Natl Acad Sci U S A. 2014 Dec 2;111(48):E5169-77. doi: 10.1073/pnas.1412663111. Epub 2014 Nov 17. Proc Natl Acad Sci U S A. 2014. PMID: 25404305 Free PMC article.
-
Critical role of IL-25 in nematode infection-induced alterations in intestinal function.J Immunol. 2010 Dec 1;185(11):6921-9. doi: 10.4049/jimmunol.1000450. Epub 2010 Oct 25. J Immunol. 2010. PMID: 20974983 Free PMC article.
-
The newest interleukins: recent additions to the ever-growing cytokine family.Vitam Horm. 2006;74:207-28. doi: 10.1016/S0083-6729(06)74008-0. Vitam Horm. 2006. PMID: 17027516 Review.
Cited by
-
Development and function of group 2 innate lymphoid cells.Curr Opin Immunol. 2013 Apr;25(2):148-55. doi: 10.1016/j.coi.2013.02.010. Epub 2013 Apr 4. Curr Opin Immunol. 2013. PMID: 23562755 Free PMC article. Review.
-
The potential roles of interleukin-25 in infectious diseases.Front Immunol. 2022 Sep 2;13:986118. doi: 10.3389/fimmu.2022.986118. eCollection 2022. Front Immunol. 2022. PMID: 36119076 Free PMC article. Review.
-
The Heterogeneity, Origins, and Impact of Migratory iILC2 Cells in Anti-helminth Immunity.Front Immunol. 2020 Jul 23;11:1594. doi: 10.3389/fimmu.2020.01594. eCollection 2020. Front Immunol. 2020. PMID: 32793230 Free PMC article. Review.
-
Interleukin-33 and its Receptor in Pulmonary Inflammatory Diseases.Crit Rev Immunol. 2015;35(6):451-61. doi: 10.1615/CritRevImmunol.2016015865. Crit Rev Immunol. 2015. PMID: 27279043 Free PMC article. Review.
-
Group 2 innate lymphoid cells: new players in human allergic diseases.J Investig Allergol Clin Immunol. 2015;25(1):1-11; quiz 2p following 11. J Investig Allergol Clin Immunol. 2015. PMID: 25898689 Free PMC article. Review.
References
-
- Moseley, T.A., D.R. Haudenschild, L. Rose, and A.H. Reddi. 2003. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 14:155–174. - PubMed
-
- Nakae, S., Y. Komiyama, A. Nambu, K. Sudo, M. Iwase, I. Homma, K. Sekikawa, M. Asano, and Y. Iwakura. 2002. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 17:375–387. - PubMed
-
- Aarvak, T., M. Chabaud, P. Miossec, and J.B. Natvig. 1999. IL-17 is produced by some proinflammatory Th1/Th0 cells but not by Th2 cells. J. Immunol. 162:1246–1251. - PubMed
-
- Matusevicius, D., P. Kivisakk, B. He, N. Kostulas, V. Ozenci, S. Fredrikson, and H. Link. 1999. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult. Scler. 5:101–104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

