Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context
- PMID: 16602827
- PMCID: PMC1420652
- DOI: 10.1371/journal.pbio.0040115
Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context
Abstract
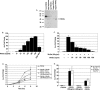
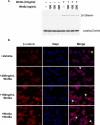
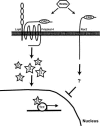
The Wnts comprise a large class of secreted proteins that control essential developmental processes such as embryonic patterning, cell growth, migration, and differentiation. In the most well-understood "canonical" Wnt signaling pathway, Wnt binding to Frizzled receptors induces beta-catenin protein stabilization and entry into the nucleus, where it complexes with T-cell factor/lymphoid enhancer factor transcription factors to affect the transcription of target genes. In addition to the canonical pathway, evidence for several other Wnt signaling pathways has accumulated, in particular for Wnt5a, which has therefore been classified as a noncanonical Wnt family member. To study the alternative mechanisms by which Wnt proteins signal, we purified the Wnt5a protein to homogeneity. We find that purified Wnt5a inhibits Wnt3a protein-induced canonical Wnt signaling in a dose-dependent manner, not by influencing beta-catenin levels but by downregulating beta-catenin-induced reporter gene expression. The Wnt5a signal is mediated by the orphan tyrosine kinase Ror2, is pertussis toxin insensitive, and does not influence cellular calcium levels. We show that in addition to its inhibitory function, Wnt5a can also activate beta-catenin signaling in the presence of the appropriate Frizzled receptor, Frizzled 4. Thus, this study shows for the first time that a single Wnt ligand can initiate discrete signaling pathways through the activation of two distinct receptors. Based on these and additional observations, we propose a model wherein receptor context dictates Wnt signaling output. In this model, signaling by different Wnt family members is not intrinsically regulated by the Wnt proteins themselves but by receptor availability.
Figures




Comment in
-
One signal, multiple pathways: diversity comes from the receptor.PLoS Biol. 2006 Apr;4(4):e131. doi: 10.1371/journal.pbio.0040131. Epub 2006 Apr 4. PLoS Biol. 2006. PMID: 20076560 Free PMC article. No abstract available.
Similar articles
-
The Wnt5a/Ror2 noncanonical signaling pathway inhibits canonical Wnt signaling in K562 cells.Int J Mol Med. 2011 Jan;27(1):63-9. doi: 10.3892/ijmm.2010.560. Epub 2010 Nov 10. Int J Mol Med. 2011. PMID: 21069266
-
Wnt5a induces homodimerization and activation of Ror2 receptor tyrosine kinase.J Cell Biochem. 2008 Oct 1;105(2):497-502. doi: 10.1002/jcb.21848. J Cell Biochem. 2008. PMID: 18615587
-
Ror2 receptor requires tyrosine kinase activity to mediate Wnt5A signaling.J Biol Chem. 2009 Oct 30;284(44):30167-76. doi: 10.1074/jbc.M109.041715. Epub 2009 Aug 31. J Biol Chem. 2009. PMID: 19720827 Free PMC article.
-
Hear the Wnt Ror: how melanoma cells adjust to changes in Wnt.Pigment Cell Melanoma Res. 2009 Dec;22(6):724-39. doi: 10.1111/j.1755-148X.2009.00627.x. Epub 2009 Aug 25. Pigment Cell Melanoma Res. 2009. PMID: 19708915 Free PMC article. Review.
-
Ror-family receptor tyrosine kinases in noncanonical Wnt signaling: their implications in developmental morphogenesis and human diseases.Dev Dyn. 2010 Jan;239(1):1-15. doi: 10.1002/dvdy.21991. Dev Dyn. 2010. PMID: 19530173 Review.
Cited by
-
Expression of Wnt5a and its receptor Fzd2 is changed in the spinal cord of adult amyotrophic lateral sclerosis transgenic mice.Int J Clin Exp Pathol. 2013 Jun 15;6(7):1245-60. Print 2013. Int J Clin Exp Pathol. 2013. PMID: 23826406 Free PMC article.
-
Combinatorial Wnt signaling landscape during brachiopod anteroposterior patterning.BMC Biol. 2024 Sep 19;22(1):212. doi: 10.1186/s12915-024-01988-w. BMC Biol. 2024. PMID: 39300453 Free PMC article.
-
Wnt/β-catenin and its diverse physiological cell signaling pathways in neurodegenerative and neuropsychiatric disorders.J Neuroimmune Pharmacol. 2012 Dec;7(4):725-30. doi: 10.1007/s11481-012-9412-x. Epub 2012 Nov 1. J Neuroimmune Pharmacol. 2012. PMID: 23114888 Free PMC article. Review.
-
Wnt secretion and gradient formation.Int J Mol Sci. 2013 Mar 1;14(3):5130-45. doi: 10.3390/ijms14035130. Int J Mol Sci. 2013. PMID: 23455472 Free PMC article.
-
Wnt5a Signaling in Cancer.Cancers (Basel). 2016 Aug 26;8(9):79. doi: 10.3390/cancers8090079. Cancers (Basel). 2016. PMID: 27571105 Free PMC article. Review.
References
-
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. - PubMed
-
- Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–1851. - PubMed
-
- Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. - PubMed
-
- Shimizu H, Julius MA, Giarre M, Zheng Z, Brown AM, et al. Transformation by Wnt family proteins correlates with regulation of beta-catenin. Cell Growth Differ. 1997;8:1349–1358. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

