Refolding hydrogels self-assembled from N-(2-hydroxypropyl)methacrylamide graft copolymers by antiparallel coiled-coil formation
- PMID: 16602737
- PMCID: PMC2529150
- DOI: 10.1021/bm051002k
Refolding hydrogels self-assembled from N-(2-hydroxypropyl)methacrylamide graft copolymers by antiparallel coiled-coil formation
Abstract
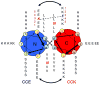
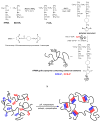
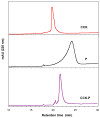
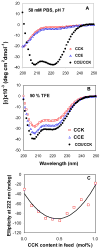
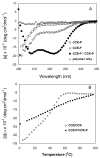
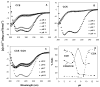
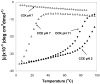
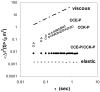
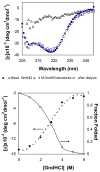
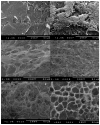
A novel hybrid hydrogel system based on N-(2-hydroxypropyl)methacrylamide copolymers was proposed. It consisted of the hydrophilic polymer backbone and a pair of oppositely charged peptide grafts. Two distinct pentaheptad peptides (CCE and CCK) were anticipated to create a dimerization motif and serve as physical cross-linkers. Consequently, the graft copolymers CCE-P and CCK-P self-assembled into hybrid hydrogels in situ; the process was modulated by the formation of antiparallel heterodimeric coiled-coils. This approach possesses an advantage to decrease the steric hindrance of the polymer backbone on the "in-register" alignment of peptide grafts. Indeed, equimolar mixtures of the graft copolymers, CCE-P/CCK-P, have been observed to self-assemble into hydrogels in PBS solution at neutral pH at concentrations as low as 0.1 wt %. Circular dichroism spectroscopy, sedimentation equilibrium experiments, and microrheology revealed that the self-assembly process corresponded to the two-stranded alpha-helical coiled-coil formation between CCE and CCK. Moreover, the formation of hybrid hydrogels was reversible. Denaturation of the coiled-coil domains with guanidine hydrochloride (GdnHCl) solutions resulted in disassembly of the hydrogels. Removal of GdnHCl by dialysis caused coiled-coil refolding and hydrogel reassembly. Scanning electron microscopy results demonstrated that the concentration of the graft copolymers had a significant impact on the structure and morphology of self-assembled hydrogels.
Figures
Similar articles
-
Dynamic light scattering study of self-assembly of HPMA hybrid graft copolymers.Biomacromolecules. 2008 Feb;9(2):510-7. doi: 10.1021/bm701001f. Epub 2008 Jan 22. Biomacromolecules. 2008. PMID: 18208316
-
Genetically engineered block copolymers: influence of the length and structure of the coiled-coil blocks on hydrogel self-assembly.Pharm Res. 2008 Mar;25(3):674-82. doi: 10.1007/s11095-007-9343-z. Epub 2007 Aug 23. Pharm Res. 2008. PMID: 17713844
-
Hybrid hydrogels self-assembled from HPMA copolymers containing peptide grafts.Macromol Biosci. 2006 Mar 14;6(3):201-9. doi: 10.1002/mabi.200500208. Macromol Biosci. 2006. PMID: 16514591
-
Coiled coils: attractive protein folding motifs for the fabrication of self-assembled, responsive and bioactive materials.Chem Soc Rev. 2010 Sep;39(9):3541-75. doi: 10.1039/b914339b. Epub 2010 Aug 2. Chem Soc Rev. 2010. PMID: 20676430 Review.
-
The coiled coils in the design of protein-based constructs: hybrid hydrogels and epitope displays.J Control Release. 2001 May 14;72(1-3):57-70. doi: 10.1016/s0168-3659(01)00262-0. J Control Release. 2001. PMID: 11389985 Review.
Cited by
-
Influence of Network Topology on the Viscoelastic Properties of Dynamically Crosslinked Hydrogels.Front Chem. 2020 Jun 30;8:536. doi: 10.3389/fchem.2020.00536. eCollection 2020. Front Chem. 2020. PMID: 32719773 Free PMC article.
-
Drug-free macromolecular therapeutics: Impact of structure on induction of apoptosis in Raji B cells.J Control Release. 2017 Oct 10;263:139-150. doi: 10.1016/j.jconrel.2016.12.025. Epub 2016 Dec 24. J Control Release. 2017. PMID: 28024916 Free PMC article.
-
Immunogenicity of coiled-coil based drug-free macromolecular therapeutics.Biomaterials. 2014 Jul;35(22):5886-96. doi: 10.1016/j.biomaterials.2014.03.063. Epub 2014 Apr 22. Biomaterials. 2014. PMID: 24767787 Free PMC article.
-
Hydrophilic biomaterials: From crosslinked and self-assembled hydrogels to polymer-drug conjugates and drug-free macromolecular therapeutics.J Control Release. 2024 Sep;373:1-22. doi: 10.1016/j.jconrel.2024.05.012. Epub 2024 May 17. J Control Release. 2024. PMID: 38734315
-
Synthesis and primary characterization of self-assembled peptide-based hydrogels.Methods Mol Biol. 2008;474:61-77. doi: 10.1007/978-1-59745-480-3_5. Methods Mol Biol. 2008. PMID: 19031061 Free PMC article. Review.
References
-
- Rajagopal K, Schneider JP. Curr Opin Struct Biol. 2004;14:480–486. - PubMed
-
- Zhang S. Biotechnol Adv. 2002;20:321–339. - PubMed
-
- MacPhee CE, Woolfson DN. Curr Opin Solid State Mater Sci. 2004;8:141–149.
-
- Vandermeulen GW, Klok HA. Macromol Biosci. 2004;4:383–398. - PubMed
-
- Lutolf MP, Hubbell JA. Nat Biotechnol. 2005;23:47–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

