GGA function is required for maturation of neuroendocrine secretory granules
- PMID: 16601685
- PMCID: PMC1440831
- DOI: 10.1038/sj.emboj.7601067
GGA function is required for maturation of neuroendocrine secretory granules
Abstract
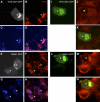
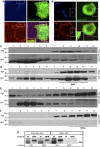
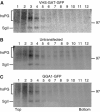
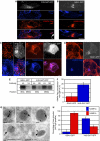
Secretory granule (SG) maturation has been proposed to involve formation of clathrin-coated vesicles (CCVs) from immature SGs (ISGs). We tested the effect of inhibiting CCV budding by using the clathrin adaptor GGA (Golgi-associated, gamma-ear-containing, ADP-ribosylation factor-binding protein) on SG maturation in neuroendocrine cells. Overexpression of a truncated, GFP-tagged GGA, VHS (Vps27, Hrs, Stam)-GAT (GGA and target of myb (TOM))-GFP led to retention of MPR, VAMP4, and syntaxin 6 in mature SGs (MSGs), suggesting that CCV budding from ISGs is inhibited by the SG-localizing VHS-GAT-GFP. Furthermore, VHS-GAT-GFP-overexpression disrupts prohormone convertase 2 (PC2) autocatalytic cleavage, processing of secretogranin II to its product p18, and the correlation between PC2 and p18 levels. All these effects were not observed if full-length GGA1-GFP was overexpressed. Neither GGA1-GFP nor VHS-GAT-GFP perturbed SG protein budding from the TGN, or homotypic fusion of ISGs. Reducing GGA3 levels by using short interfering (si)RNA also led to VAMP4 retention in SGs, and inhibition of PC2 activity. Our results suggest that inhibition of CCV budding from ISGs downregulates the sorting from the ISGs and perturbs the intragranular activity of PC2.
Figures
Similar articles
-
Synaptotagmin IV is necessary for the maturation of secretory granules in PC12 cells.J Cell Biol. 2006 Apr 24;173(2):241-51. doi: 10.1083/jcb.200506163. Epub 2006 Apr 17. J Cell Biol. 2006. PMID: 16618809 Free PMC article.
-
Intracellular trafficking and secretion of adiponectin is dependent on GGA-coated vesicles.J Biol Chem. 2006 Mar 17;281(11):7253-9. doi: 10.1074/jbc.M511313200. Epub 2005 Dec 29. J Biol Chem. 2006. PMID: 16407204
-
Homotypic fusion of immature secretory granules during maturation requires syntaxin 6.Mol Biol Cell. 2001 Jun;12(6):1699-709. doi: 10.1091/mbc.12.6.1699. Mol Biol Cell. 2001. PMID: 11408578 Free PMC article.
-
Secretory granule biogenesis: rafting to the SNARE.Trends Cell Biol. 2001 Mar;11(3):116-22. doi: 10.1016/s0962-8924(00)01907-3. Trends Cell Biol. 2001. PMID: 11306272 Review.
-
The GGA proteins: key players in protein sorting at the trans-Golgi network.Eur J Cell Biol. 2004 Jul;83(6):257-62. doi: 10.1078/0171-9335-00374. Eur J Cell Biol. 2004. PMID: 15511083 Review.
Cited by
-
Roles of myosin Va and Rab3D in membrane remodeling of immature secretory granules.Cell Mol Neurobiol. 2010 Nov;30(8):1303-8. doi: 10.1007/s10571-010-9597-6. Epub 2010 Nov 16. Cell Mol Neurobiol. 2010. PMID: 21080055 Free PMC article.
-
HID-1, a new component of the peptidergic signaling pathway.Genetics. 2011 Feb;187(2):467-83. doi: 10.1534/genetics.110.121996. Epub 2010 Nov 29. Genetics. 2011. PMID: 21115972 Free PMC article.
-
Arf GTPase-activating proteins SMAP1 and AGFG2 regulate the size of Weibel-Palade bodies and exocytosis of von Willebrand factor.Biol Open. 2021 Sep 15;10(9):bio058789. doi: 10.1242/bio.058789. Epub 2021 Sep 1. Biol Open. 2021. PMID: 34369554 Free PMC article.
-
Rab3D is critical for secretory granule maturation in PC12 cells.PLoS One. 2013;8(3):e57321. doi: 10.1371/journal.pone.0057321. Epub 2013 Mar 19. PLoS One. 2013. PMID: 23526941 Free PMC article.
-
Cdc42 controls secretory granules morphology in rodent salivary glands in vivo.Commun Integr Biol. 2020 Feb 11;13(1):22-26. doi: 10.1080/19420889.2020.1724605. eCollection 2020. Commun Integr Biol. 2020. PMID: 32128025 Free PMC article.
References
-
- Austin C, Hinners I, Tooze SA (2000) Direct and GTP-dependent interaction of ADP-ribosylation Factor 1 with clathrin adaptor protein AP-1 on immature secretory granules. J Biol Chem 275: 21862–21869 - PubMed
-
- Barr F, Huttner WB (1996) A role for ADP-ribosylation factor1, but not COP I, in secretory vesicle biogenesis from the trans-Golgi network. FEBS Lett 384: 65–70 - PubMed
-
- Bonifacino JS (2004) The GGA proteins: adaptors on the move. Nat Rev Mol Cell Biol 5: 23–32 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

