Spinal cord endoplasmic reticulum stress associated with a microsomal accumulation of mutant superoxide dismutase-1 in an ALS model
- PMID: 16595634
- PMCID: PMC1458691
- DOI: 10.1073/pnas.0509227103
Spinal cord endoplasmic reticulum stress associated with a microsomal accumulation of mutant superoxide dismutase-1 in an ALS model
Abstract
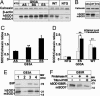
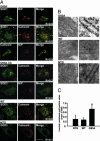
Mutation in superoxide dismutase-1 (SOD1), which is a cause of ALS, alters the folding patterns of this protein. Accumulation of misfolded mutant SOD1 might activate endoplasmic reticulum (ER) stress pathways. Here we show that transgenic mice expressing ALS-linked SOD1 mutants exhibit molecular alterations indicative of a recruitment of ER's signaling machinery. We demonstrate by biochemical and morphological methods that mutant SOD1 accumulates inside the ER, where it forms insoluble high molecular weight species and interacts with the ER chaperone immunoglobulin-binding protein. These alterations are age- and region-specific, because they develop over the course of the disease and occur in the affected spinal cord but not in the nonaffected cerebellum in transgenic mutant SOD1 mice. Our results suggest a toxic mechanism for mutant SOD1 by which this ubiquitously expressed pathogenic protein could affect motor neuron survival and contribute to the selective motor neuronal degeneration in ALS.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures

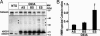

Similar articles
-
The endoplasmic reticulum-Golgi pathway is a target for translocation and aggregation of mutant superoxide dismutase linked to ALS.FASEB J. 2008 Jul;22(7):2476-87. doi: 10.1096/fj.07-092783. Epub 2008 Mar 12. FASEB J. 2008. PMID: 18337461
-
Mutant SOD1 linked to familial amyotrophic lateral sclerosis, but not wild-type SOD1, induces ER stress in COS7 cells and transgenic mice.Biochem Biophys Res Commun. 2003 Apr 4;303(2):496-503. doi: 10.1016/s0006-291x(03)00353-x. Biochem Biophys Res Commun. 2003. PMID: 12659845
-
Superoxide dismutase 1 mutants related to amyotrophic lateral sclerosis induce endoplasmic stress in neuro2a cells.J Neurochem. 2008 Feb;104(4):993-1005. doi: 10.1111/j.1471-4159.2007.05053.x. J Neurochem. 2008. PMID: 18233996
-
Transgenic mouse model for familial amyotrophic lateral sclerosis with superoxide dismutase-1 mutation.Neuropathology. 2001 Mar;21(1):82-92. doi: 10.1046/j.1440-1789.2001.00361.x. Neuropathology. 2001. PMID: 11304046 Review.
-
Stress signaling from the endoplasmic reticulum: A central player in the pathogenesis of amyotrophic lateral sclerosis.IUBMB Life. 2011 Sep;63(9):754-63. doi: 10.1002/iub.520. Epub 2011 Aug 10. IUBMB Life. 2011. PMID: 21834058 Review.
Cited by
-
Reduced calreticulin levels link endoplasmic reticulum stress and Fas-triggered cell death in motoneurons vulnerable to ALS.J Neurosci. 2012 Apr 4;32(14):4901-12. doi: 10.1523/JNEUROSCI.5431-11.2012. J Neurosci. 2012. PMID: 22492046 Free PMC article.
-
Potential roles of the endoplasmic reticulum stress pathway in amyotrophic lateral sclerosis.Front Aging Neurosci. 2023 Feb 15;15:1047897. doi: 10.3389/fnagi.2023.1047897. eCollection 2023. Front Aging Neurosci. 2023. PMID: 36875699 Free PMC article. Review.
-
Towards Understanding the Relationship Between ER Stress and Unfolded Protein Response in Amyotrophic Lateral Sclerosis.Front Aging Neurosci. 2022 Jun 15;14:892518. doi: 10.3389/fnagi.2022.892518. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35783140 Free PMC article. Review.
-
The homocysteine-inducible endoplasmic reticulum (ER) stress protein Herp counteracts mutant α-synuclein-induced ER stress via the homeostatic regulation of ER-resident calcium release channel proteins.Hum Mol Genet. 2012 Mar 1;21(5):963-77. doi: 10.1093/hmg/ddr502. Epub 2011 Nov 1. Hum Mol Genet. 2012. PMID: 22045699 Free PMC article.
-
Different regulation of wild-type and mutant Cu,Zn superoxide dismutase localization in mammalian mitochondria.Hum Mol Genet. 2008 Nov 1;17(21):3303-17. doi: 10.1093/hmg/ddn226. Epub 2008 Aug 13. Hum Mol Genet. 2008. PMID: 18703498 Free PMC article.
References
-
- Julien J. P. Cell. 2001;104:581–591. - PubMed
-
- Bruijn L. I., Houseweart M. K., Kato S., Anderson K. L., Anderson S. D., Ohama E., Reaume A. G., Scott R. W., Cleveland D. W. Science. 1998;281:1851–1854. - PubMed
-
- Bruijn L. I., Becher M. W., Lee M. K., Anderson K. L., Jenkins N. A., Copeland N. G., Sisodia S., Rothstein J. D., Borchelt D. R., Price D. L., et al. Neuron. 1997;18:327–338. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

