Alpha1-adrenergic receptors prevent a maladaptive cardiac response to pressure overload
- PMID: 16585965
- PMCID: PMC1421341
- DOI: 10.1172/JCI22811
Alpha1-adrenergic receptors prevent a maladaptive cardiac response to pressure overload
Abstract
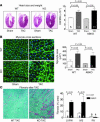
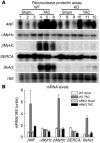
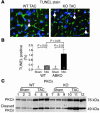
An alpha1-adrenergic receptor (alpha1-AR) antagonist increased heart failure in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT), but it is unknown whether this adverse result was due to alpha1-AR inhibition or a nonspecific drug effect. We studied cardiac pressure overload in mice with double KO of the 2 main alpha1-AR subtypes in the heart, alpha 1A (Adra1a) and alpha 1B (Adra1b). At 2 weeks after transverse aortic constriction (TAC), KO mouse survival was only 60% of WT, and surviving KO mice had lower ejection fractions and larger end-diastolic volumes than WT mice. Mechanistically, final heart weight and myocyte cross-sectional area were the same after TAC in KO and WT mice. However, KO hearts after TAC had increased interstitial fibrosis, increased apoptosis, and failed induction of the fetal hypertrophic genes. Before TAC, isolated KO myocytes were more susceptible to apoptosis after oxidative and beta-AR stimulation, and beta-ARs were desensitized. Thus, alpha1-AR deletion worsens dilated cardiomyopathy after pressure overload, by multiple mechanisms, indicating that alpha1-signaling is required for cardiac adaptation. These results suggest that the adverse cardiac effects of alpha1-antagonists in clinical trials are due to loss of alpha1-signaling in myocytes, emphasizing concern about clinical use of alpha1-antagonists, and point to a revised perspective on sympathetic activation in heart failure.
Figures
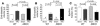


Comment in
-
Cardiac 7-transmembrane-spanning domain receptor portfolios: diversify, diversify, diversify.J Clin Invest. 2006 Apr;116(4):875-7. doi: 10.1172/JCI28234. J Clin Invest. 2006. PMID: 16585959 Free PMC article.
Similar articles
-
An alpha1A-adrenergic-extracellular signal-regulated kinase survival signaling pathway in cardiac myocytes.Circulation. 2007 Feb 13;115(6):763-72. doi: 10.1161/CIRCULATIONAHA.106.664862. Epub 2007 Feb 5. Circulation. 2007. PMID: 17283256
-
Adverse effects of constitutively active alpha(1B)-adrenergic receptors after pressure overload in mouse hearts.Am J Physiol Heart Circ Physiol. 2000 Sep;279(3):H1079-86. doi: 10.1152/ajpheart.2000.279.3.H1079. Am J Physiol Heart Circ Physiol. 2000. PMID: 10993770
-
Alpha-calcitonin gene-related peptide is protective against pressure overload-induced heart failure.Regul Pept. 2013 Aug 10;185:20-8. doi: 10.1016/j.regpep.2013.06.008. Epub 2013 Jun 28. Regul Pept. 2013. PMID: 23816470
-
Cardiac α1A-adrenergic receptors: emerging protective roles in cardiovascular diseases.Am J Physiol Heart Circ Physiol. 2021 Feb 1;320(2):H725-H733. doi: 10.1152/ajpheart.00621.2020. Epub 2020 Dec 4. Am J Physiol Heart Circ Physiol. 2021. PMID: 33275531 Free PMC article. Review.
-
Cardiac alpha1-adrenergic receptors: novel aspects of expression, signaling mechanisms, physiologic function, and clinical importance.Pharmacol Rev. 2013 Dec 24;66(1):308-33. doi: 10.1124/pr.112.007203. Print 2014. Pharmacol Rev. 2013. PMID: 24368739 Free PMC article. Review.
Cited by
-
Alpha-1-adrenergic receptors in heart failure: the adaptive arm of the cardiac response to chronic catecholamine stimulation.J Cardiovasc Pharmacol. 2014 Apr;63(4):291-301. doi: 10.1097/FJC.0000000000000032. J Cardiovasc Pharmacol. 2014. PMID: 24145181 Free PMC article. Review.
-
Nuclear alpha1-adrenergic receptors signal activated ERK localization to caveolae in adult cardiac myocytes.Circ Res. 2008 Oct 24;103(9):992-1000. doi: 10.1161/CIRCRESAHA.108.176024. Epub 2008 Sep 18. Circ Res. 2008. PMID: 18802028 Free PMC article.
-
CRISPLD1: a novel conserved target in the transition to human heart failure.Basic Res Cardiol. 2020 Mar 7;115(3):27. doi: 10.1007/s00395-020-0784-4. Basic Res Cardiol. 2020. PMID: 32146539 Free PMC article.
-
How reliable are G-protein-coupled receptor antibodies?Naunyn Schmiedebergs Arch Pharmacol. 2009 Apr;379(4):385-8. doi: 10.1007/s00210-009-0395-y. Epub 2009 Jan 27. Naunyn Schmiedebergs Arch Pharmacol. 2009. PMID: 19172248
-
Fit-for-purpose based testing and validation of antibodies to amino- and carboxy-terminal domains of cannabinoid receptor 1.Histochem Cell Biol. 2021 Nov;156(5):479-502. doi: 10.1007/s00418-021-02025-5. Epub 2021 Aug 27. Histochem Cell Biol. 2021. PMID: 34453219 Free PMC article.
References
-
- McCloskey D.T., et al. Abnormal myocardial contraction in alpha(1a)- and alpha(1b)-adrenoceptor double-knockout mice. J. Mol. Cell. Cardiol. 2003;35:1207–1216. - PubMed
-
- Mani K., Ashton A.W., Kitsis R.N. Taking the bad out of adrenergic stimulation. J. Mol. Cell. Cardiol. 2002;34:709–712. - PubMed
-
- Salvi S. Protecting the myocardium from ischemic injury: a critical role for alpha(1)-adrenoreceptors? Chest. 2001;119:1242–1249. - PubMed
-
- ALLHAT Collaborative Research Group. scular events in hypertensive patients randomized to doxazosin vs chlorthalidone: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). JAMA. 283:1967–1975. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

