Trim-cyclophilin A fusion proteins can restrict human immunodeficiency virus type 1 infection at two distinct phases in the viral life cycle
- PMID: 16571822
- PMCID: PMC1440439
- DOI: 10.1128/JVI.80.8.4061-4067.2006
Trim-cyclophilin A fusion proteins can restrict human immunodeficiency virus type 1 infection at two distinct phases in the viral life cycle
Abstract
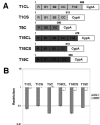
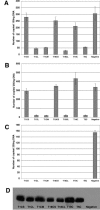
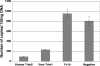
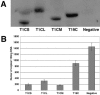
The Trim5alpha protein from several primates restricts retroviruses in a capsid (CA)-dependent manner. In owl monkeys, the B30.2 domain of Trim5 has been replaced by cyclophilin A (CypA) following a retrotransposition. Restriction of human immunodeficiency virus type 1 (HIV-1) by the resulting Trim5-CypA fusion protein depends on CA binding to CypA, suggesting both that the B30.2 domain might be involved in CA binding and that the tripartite RING motif, B-BOX, and coiled coil (RBCC) motif domain can function independently of the B30.2 domain in restriction. To investigate the potential of RBCCs from other Trims to participate in restricting HIV-1, CypA was fused to the RBCC of Trim1, Trim18, and Trim19 and tested for restriction. Despite low identity within the RBCC domain, all fusion proteins were found to restrict HIV-1 but not the nonbinding G89V mutant, indicating that the overall structure of RBCC and not its primary sequence was important for the restriction function. The critical interaction between CA and Trim-CypA appears to take place soon after viral entry. Quantitative PCR analysis of viral reverse transcriptase products revealed that the different fusion proteins block HIV-1 at two distinct stages of its life cycle, either prior to reverse transcription or just before integration. With Trim1 and Trim18, this timing is dependent on the length of the Trim component of the fusion protein. These observations suggest that restriction factor binding can have different mechanistic consequences.
Figures

Similar articles
-
Cyclophilin A renders human immunodeficiency virus type 1 sensitive to Old World monkey but not human TRIM5 alpha antiviral activity.J Virol. 2006 May;80(10):4683-90. doi: 10.1128/JVI.80.10.4683-4690.2006. J Virol. 2006. PMID: 16641261 Free PMC article.
-
Evolution of a TRIM5-CypA splice isoform in old world monkeys.PLoS Pathog. 2008 Feb 29;4(2):e1000003. doi: 10.1371/journal.ppat.1000003. PLoS Pathog. 2008. PMID: 18389077 Free PMC article.
-
Cyclophilin A protects HIV-1 from restriction by human TRIM5α.Nat Microbiol. 2019 Dec;4(12):2044-2051. doi: 10.1038/s41564-019-0592-5. Epub 2019 Oct 21. Nat Microbiol. 2019. PMID: 31636416 Free PMC article.
-
The control of viral infection by tripartite motif proteins and cyclophilin A.Retrovirology. 2007 Jun 12;4:40. doi: 10.1186/1742-4690-4-40. Retrovirology. 2007. PMID: 17565686 Free PMC article. Review.
-
Retroviral restriction factors TRIM5α: therapeutic strategy to inhibit HIV-1 replication.Curr Med Chem. 2011;18(17):2649-54. doi: 10.2174/092986711795933687. Curr Med Chem. 2011. PMID: 21568899 Review.
Cited by
-
Evidence for biphasic uncoating during HIV-1 infection from a novel imaging assay.Retrovirology. 2013 Jul 9;10:70. doi: 10.1186/1742-4690-10-70. Retrovirology. 2013. PMID: 23835323 Free PMC article.
-
The innate immune roles of host factors TRIM5α and Cyclophilin A on HIV-1 replication.Med Microbiol Immunol. 2015 Oct;204(5):557-65. doi: 10.1007/s00430-015-0417-y. Epub 2015 Apr 19. Med Microbiol Immunol. 2015. PMID: 25894765 Review.
-
Both TRIM5alpha and TRIMCyp have only weak antiviral activity in canine D17 cells.Retrovirology. 2007 Sep 24;4:68. doi: 10.1186/1742-4690-4-68. Retrovirology. 2007. PMID: 17892575 Free PMC article.
-
Transportin 3 promotes a nuclear maturation step required for efficient HIV-1 integration.PLoS Pathog. 2011 Aug;7(8):e1002194. doi: 10.1371/journal.ppat.1002194. Epub 2011 Aug 25. PLoS Pathog. 2011. PMID: 21901095 Free PMC article.
-
Viral and cellular requirements for the nuclear entry of retroviral preintegration nucleoprotein complexes.Viruses. 2013 Oct 7;5(10):2483-511. doi: 10.3390/v5102483. Viruses. 2013. PMID: 24103892 Free PMC article. Review.
References
-
- Bainbridge, J. W., C. Stephens, K. Parsley, C. Demaison, A. Halfyard, A. J. Thrasher, and R. R. Ali. 2001. In vivo gene transfer to the mouse eye using an HIV-based lentiviral vector; efficient long-term transduction of corneal endothelium and retinal pigment epithelium. Gene Ther. 8:1665-1668. - PubMed
-
- Boutell, C., and R. D. Everett. 2003. The herpes simplex virus type 1 (HSV-1) regulatory protein ICP0 interacts with and ubiquitinates p53. J. Biol. Chem. 278:36596-36602. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

