Nanodisk-associated amphotericin B clears Leishmania major cutaneous infection in susceptible BALB/c mice
- PMID: 16569834
- PMCID: PMC1426947
- DOI: 10.1128/AAC.50.4.1238-1244.2006
Nanodisk-associated amphotericin B clears Leishmania major cutaneous infection in susceptible BALB/c mice
Abstract
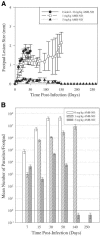
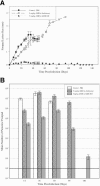
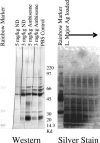
Nanometer-scale, apolipoprotein-stabilized phospholipid bilayer disk complexes (nanodisks [ND]) harboring the toxic and poorly soluble antileishmanial agent amphotericin B (AMB) were examined for efficacy in treatment of Leishmania major-infected BALB/c mice (Mus musculus). L. major-infected mice were intraperitoneally (i.p.) treated with AMB-ND in 0-, 1-, and 5-mg/kg doses at 24 h, 48 h, and 4, 7, 14, and 21 days postinfection in two experiments. L. major-infected mice were i.p. treated with phosphate-buffered saline, 5 mg/kg AMB-ND, or 5 mg/kg lipid-associated amphotericin B (liposomal amphotericin B, AmBisome) at 24 h, 48 h, and 10, 20, 30, and 40 days postinfection in one experiment. Parasite numbers, footpad lesion size progression, and development of cytokine responses were assayed at days 7, 15, 30, 50, 140, and 250 or at days 14, 30, 50, 95, and 140 postinfection. Mice administered AMB-ND in 1- or 5-mg/kg doses were significantly protected from L. major, displaying decreases in lesion size and parasite burden, particularly at the 5-mg/kg dosage level. In contrast to the i.p. treated AmBisome group, BALB/c mice treated with i.p. AMB-ND completely cleared an L. major infection by 140 to 250 days postinfection, with no lesions remaining and no parasites isolated from infected animals. Restimulated mixed lymphocyte culture cytokine responses (interleukin-4 [IL-4], IL-12, IL-10, NO, and gamma interferon) were unchanged by AMB-ND administration compared to controls. The marked clearance of Leishmania parasites from a susceptible strain of mice without an appreciable change in the cytokine response suggests that AMB-ND represent a potentially useful formulation for treatment of intrahistiocytic organisms.
Figures
Similar articles
-
Biodistribution and In Vivo Antileishmanial Activity of 1,2-Distigmasterylhemisuccinoyl-sn-Glycero-3-Phosphocholine Liposome-Intercalated Amphotericin B.Antimicrob Agents Chemother. 2017 Aug 24;61(9):e02525-16. doi: 10.1128/AAC.02525-16. Print 2017 Sep. Antimicrob Agents Chemother. 2017. PMID: 28630182 Free PMC article.
-
Effects of Amphotericin B-Conjugated Functionalized Carbon Nanoparticles in the Treatment of Cutaneous Leishmaniasis.Parasite Immunol. 2024 Oct;46(10):e13068. doi: 10.1111/pim.13068. Parasite Immunol. 2024. PMID: 39363635
-
An amphotericin B-based drug for treating experimental Leishmania major infection.Trans R Soc Trop Med Hyg. 2010 Nov;104(11):749-50. doi: 10.1016/j.trstmh.2010.08.010. Epub 2010 Sep 17. Trans R Soc Trop Med Hyg. 2010. PMID: 20850850 Free PMC article.
-
Development of a topical liposomal formulation of Amphotericin B for the treatment of cutaneous leishmaniasis.Int J Parasitol Drugs Drug Resist. 2019 Dec;11:156-165. doi: 10.1016/j.ijpddr.2019.09.004. Epub 2019 Sep 23. Int J Parasitol Drugs Drug Resist. 2019. PMID: 31582344 Free PMC article.
-
Nanodisks: hydrophobic drug delivery vehicles.Expert Opin Drug Deliv. 2008 Mar;5(3):343-51. doi: 10.1517/17425247.5.3.343. Expert Opin Drug Deliv. 2008. PMID: 18318655 Review.
Cited by
-
Reconstituted HDL as a therapeutic delivery device.Biochim Biophys Acta Mol Cell Biol Lipids. 2021 Nov;1866(11):159025. doi: 10.1016/j.bbalip.2021.159025. Epub 2021 Aug 8. Biochim Biophys Acta Mol Cell Biol Lipids. 2021. PMID: 34375767 Free PMC article. Review.
-
Nanoparticulate drug delivery systems for the treatment of neglected tropical protozoan diseases.J Venom Anim Toxins Incl Trop Dis. 2019 Feb 11;25:e144118. doi: 10.1590/1678-9199-JVATITD-1441-18. eCollection 2019. J Venom Anim Toxins Incl Trop Dis. 2019. PMID: 31130996 Free PMC article.
-
Anti-leishmanial activity of Brevinin 2R and its Lauric acid conjugate type against L. major: In vitro mechanism of actions and in vivo treatment potentials.PLoS Negl Trop Dis. 2019 Feb 27;13(2):e0007217. doi: 10.1371/journal.pntd.0007217. eCollection 2019 Feb. PLoS Negl Trop Dis. 2019. PMID: 30811391 Free PMC article.
-
Nanodiscs in Membrane Biochemistry and Biophysics.Chem Rev. 2017 Mar 22;117(6):4669-4713. doi: 10.1021/acs.chemrev.6b00690. Epub 2017 Feb 8. Chem Rev. 2017. PMID: 28177242 Free PMC article. Review.
-
Proteasome inhibition for treatment of leishmaniasis, Chagas disease and sleeping sickness.Nature. 2016 Sep 8;537(7619):229-233. doi: 10.1038/nature19339. Epub 2016 Aug 8. Nature. 2016. PMID: 27501246 Free PMC article.
References
-
- Basu, M. K., and S. Lala. 2004. Macrophage specific drug delivery in experimental leishmaniasis. Curr. Mol. Med. 4:681-689. - PubMed
-
- Brito, M. E., M. G. Mendonca, Y. M. Gomes, M. L. Jardim, and F. G. Abath. 2001. Dynamics of the antibody response in patients with therapeutic or spontaneous cure of American cutaneous leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 95:203-206. - PubMed
-
- Broz, P., S. M. Benito, C. Saw, P. Burger, H. Heider, M. Pfisterer, S. Marsch, W. Meier, and P. Hunziker. 2005. Cell targeting by a generic receptor-targeted polymer nanocontainer platform. J. Cont. Release 102:475-488. - PubMed
-
- Croft, S. L., and G. H. Coombs. 2003. Leishmaniasis—current chemotherapy and recent advances in the search for novel drugs. Trends Parasitol. 19:502-508. - PubMed
-
- Datta, N., S. Mukherjee, L. Das, and P. K. Das. 2003. Targeting of immunostimulatory DNA cures experimental visceral leishmaniasis through nitric oxide up-regulation and T cell activation. Eur. J. Immunol. 33:1508-1518. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

