Crystal structures of the kainate receptor GluR5 ligand binding core dimer with novel GluR5-selective antagonists
- PMID: 16540562
- PMCID: PMC6673968
- DOI: 10.1523/JNEUROSCI.0123-06.2005
Crystal structures of the kainate receptor GluR5 ligand binding core dimer with novel GluR5-selective antagonists
Abstract
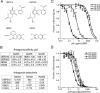
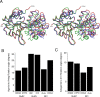
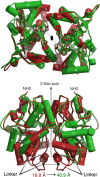
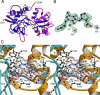
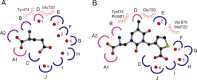
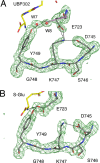
Glutamate receptor (GluR) ion channels mediate fast synaptic transmission in the mammalian CNS. Numerous crystallographic studies, the majority on the GluR2-subtype AMPA receptor, have revealed the structural basis for binding of subtype-specific agonists. In contrast, because there are far fewer antagonist-bound structures, the mechanisms for antagonist binding are much less well understood, particularly for kainate receptors that exist as multiple subtypes with a distinct biology encoded by the GluR5-7, KA1, and KA2 genes. We describe here high-resolution crystal structures for the GluR5 ligand-binding core complex with UBP302 and UBP310, novel GluR5-selective antagonists. The crystal structures reveal the structural basis for the high selectivity for GluR5 observed in radiolabel displacement assays for the isolated ligand binding cores of the GluR2, GluR5, and GluR6 subunits and during inhibition of glutamate-activated currents in studies on full-length ion channels. The antagonists bind via a novel mechanism and do not form direct contacts with the E723 side chain as occurs in all previously solved AMPA and kainate receptor agonist and antagonist complexes. This results from a hyperextension of the ligand binding core compared with previously solved structures. As a result, in dimer assemblies, there is a 22 A extension of the ion channel linkers in the transition from antagonist- to glutamate-bound forms. This large conformational change is substantially different from that described for AMPA receptors, was not possible to predict from previous work, and suggests that glutamate receptors are capable of much larger movements than previously thought.
Figures

Similar articles
-
Crystal structures of the GluR5 and GluR6 ligand binding cores: molecular mechanisms underlying kainate receptor selectivity.Neuron. 2005 Feb 17;45(4):539-52. doi: 10.1016/j.neuron.2005.01.031. Neuron. 2005. PMID: 15721240
-
Tetrazolyl isoxazole amino acids as ionotropic glutamate receptor antagonists: synthesis, modelling and molecular pharmacology.Bioorg Med Chem. 2005 Sep 15;13(18):5391-8. doi: 10.1016/j.bmc.2005.06.024. Bioorg Med Chem. 2005. PMID: 16043357
-
The structure of a mixed GluR2 ligand-binding core dimer in complex with (S)-glutamate and the antagonist (S)-NS1209.J Mol Biol. 2006 Apr 7;357(4):1184-201. doi: 10.1016/j.jmb.2006.01.024. Epub 2006 Jan 31. J Mol Biol. 2006. PMID: 16483599
-
New developments in the molecular pharmacology of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate and kainate receptors.Pharmacol Ther. 1996;70(1):65-89. doi: 10.1016/0163-7258(96)00014-9. Pharmacol Ther. 1996. PMID: 8804111 Review.
-
The biochemistry, ultrastructure, and subunit assembly mechanism of AMPA receptors.Mol Neurobiol. 2010 Dec;42(3):161-84. doi: 10.1007/s12035-010-8149-x. Epub 2010 Nov 16. Mol Neurobiol. 2010. PMID: 21080238 Free PMC article. Review.
Cited by
-
Exploring kainate receptor pharmacology using molecular dynamics simulations.Neuropharmacology. 2010 Feb;58(2):515-27. doi: 10.1016/j.neuropharm.2009.08.019. Epub 2009 Sep 6. Neuropharmacology. 2010. PMID: 19737573 Free PMC article.
-
The neurobiologist's guide to structural biology: a primer on why macromolecular structure matters and how to evaluate structural data.Neuron. 2007 May 24;54(4):511-33. doi: 10.1016/j.neuron.2007.04.026. Neuron. 2007. PMID: 17521566 Free PMC article. Review.
-
Glutamatergic mechanisms for speed control and network operation in the rodent locomotor CpG.Front Neural Circuits. 2010 Aug 6;4:19. doi: 10.3389/fncir.2010.00019. eCollection 2010. Front Neural Circuits. 2010. PMID: 20844601 Free PMC article.
-
Conformational changes at the agonist binding domain of the N-methyl-D-aspartic acid receptor.J Biol Chem. 2011 May 13;286(19):16953-7. doi: 10.1074/jbc.M111.224576. Epub 2011 Mar 24. J Biol Chem. 2011. PMID: 21454656 Free PMC article.
-
Structure, Function, and Regulation of the Kainate Receptor.Subcell Biochem. 2022;99:317-350. doi: 10.1007/978-3-031-00793-4_10. Subcell Biochem. 2022. PMID: 36151381 Review.
References
-
- Anslyn EV, Dougherty DA (2005). In: Modern physical organic chemistry Sausalito, CA: University Science Books.
-
- Arinaminpathy Y, Sansom MS, Biggin PC (2006). Binding site flexibility: molecular simulation of partial and full agonists with a glutamate receptor. Mol Pharmacol 69:5–12. - PubMed
-
- Armstrong N, Gouaux E (2000). Mechanisms for activation and antagonism of an AMPA-sensitive glutamate receptor: crystal structures of the GluR2 ligand binding core. Neuron 28:165–181. - PubMed
-
- Brauner-Osborne H, Egebjerg J, Nielsen EO, Madsen U, Krogsgaard-Larsen P (2000). Ligands for glutamate receptors: design and therapeutic prospects. J Med Chem 43:2609–2645. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
