The NEMO mutation creating the most-upstream premature stop codon is hypomorphic because of a reinitiation of translation
- PMID: 16532398
- PMCID: PMC1424680
- DOI: 10.1086/501532
The NEMO mutation creating the most-upstream premature stop codon is hypomorphic because of a reinitiation of translation
Abstract
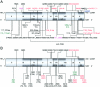
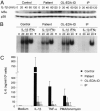
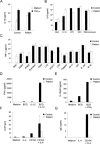
Amorphic mutations in the NF- kappa B essential modulator (NEMO) cause X-dominant incontinentia pigmenti, which is lethal in males in utero, whereas hypomorphic mutations cause X-recessive anhidrotic ectodermal dysplasia with immunodeficiency, a complex developmental disorder and life-threatening primary immunodeficiency. We characterized the NEMO mutation 110_111insC, which creates the most-upstream premature translation termination codon (at codon position 49) of any known NEMO mutation. Surprisingly, this mutation is associated with a pure immunodeficiency. We solve this paradox by showing that a Kozakian methionine codon located immediately downstream from the insertion allows the reinitiation of translation. The residual production of an NH(2)-truncated NEMO protein was sufficient for normal fetal development and for the subsequent normal development of skin appendages but was insufficient for the development of protective immune responses.
Figures


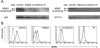

Similar articles
-
Immunodeficiency in Two Female Patients with Incontinentia Pigmenti with Heterozygous NEMO Mutation Diagnosed by LPS Unresponsiveness.J Clin Immunol. 2017 Aug;37(6):529-538. doi: 10.1007/s10875-017-0417-3. Epub 2017 Jul 12. J Clin Immunol. 2017. PMID: 28702714
-
Novel hypomorphic mutation in IKBKG impairs NEMO-ubiquitylation causing ectodermal dysplasia, immunodeficiency, incontinentia pigmenti, and immune thrombocytopenic purpura.Clin Immunol. 2015 Oct;160(2):163-71. doi: 10.1016/j.clim.2015.06.007. Epub 2015 Jun 24. Clin Immunol. 2015. PMID: 26117626
-
Insight into IKBKG/NEMO locus: report of new mutations and complex genomic rearrangements leading to incontinentia pigmenti disease.Hum Mutat. 2014 Feb;35(2):165-77. doi: 10.1002/humu.22483. Epub 2013 Dec 12. Hum Mutat. 2014. PMID: 24339369
-
EDA-ID and IP, two faces of the same coin: how the same IKBKG/NEMO mutation affecting the NF-κB pathway can cause immunodeficiency and/or inflammation.Int Rev Immunol. 2015;34(6):445-59. doi: 10.3109/08830185.2015.1055331. Epub 2015 Aug 13. Int Rev Immunol. 2015. PMID: 26269396 Review.
-
The NF-kappaB signalling pathway in human diseases: from incontinentia pigmenti to ectodermal dysplasias and immune-deficiency syndromes.Hum Mol Genet. 2002 Oct 1;11(20):2371-5. doi: 10.1093/hmg/11.20.2371. Hum Mol Genet. 2002. PMID: 12351572 Review.
Cited by
-
TLR3 immunity to infection in mice and humans.Curr Opin Immunol. 2013 Feb;25(1):19-33. doi: 10.1016/j.coi.2012.11.001. Epub 2013 Jan 3. Curr Opin Immunol. 2013. PMID: 23290562 Free PMC article. Review.
-
N-terminal proteomics and ribosome profiling provide a comprehensive view of the alternative translation initiation landscape in mice and men.Mol Cell Proteomics. 2014 May;13(5):1245-61. doi: 10.1074/mcp.M113.036442. Epub 2014 Mar 12. Mol Cell Proteomics. 2014. PMID: 24623590 Free PMC article.
-
MMADHC premature termination codons in the pathogenesis of cobalamin D disorder: Potential of translational readthrough reconstitution.Mol Genet Metab Rep. 2021 Jan 27;26:100710. doi: 10.1016/j.ymgmr.2021.100710. eCollection 2021 Mar. Mol Genet Metab Rep. 2021. PMID: 33552904 Free PMC article.
-
Early LQT2 nonsense mutation generates N-terminally truncated hERG channels with altered gating properties by the reinitiation of translation.J Mol Cell Cardiol. 2012 Nov;53(5):725-33. doi: 10.1016/j.yjmcc.2012.08.021. Epub 2012 Sep 3. J Mol Cell Cardiol. 2012. PMID: 22964610 Free PMC article.
-
Nonsense mutation-dependent reinitiation of translation in mammalian cells.Nucleic Acids Res. 2019 Jul 9;47(12):6330-6338. doi: 10.1093/nar/gkz319. Nucleic Acids Res. 2019. PMID: 31045216 Free PMC article.
References
Web Resources
-
- Prediction of Translation Initiation ATG, http://www.hri.co.jp/atgpr/
References
-
- Abinun M, Spickett G, Appleton AL, Flood T, Cant AJ (1996) Anhidrotic ectodermal dysplasia associated with specific antibody deficiency. Eur J Pediatr 155:146–147 - PubMed
-
- Aradhya S, Woffendin H, Jakins T, Bardaro T, Esposito T, Smahi A, Shaw C, Levy M, Munnich A, D’Urso M, Lewis RA, Kenwrick S, Nelson DL (2001b) A recurrent deletion in the ubiquitously expressed NEMO (IKK-gamma) gene accounts for the vast majority of incontinentia pigmenti mutations. Hum Mol Genet 10:2171–217910.1093/hmg/10.19.2171 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

