The D-type cyclin CYCD3;1 is limiting for the G1-to-S-phase transition in Arabidopsis
- PMID: 16517759
- PMCID: PMC1425856
- DOI: 10.1105/tpc.105.039636
The D-type cyclin CYCD3;1 is limiting for the G1-to-S-phase transition in Arabidopsis
Abstract
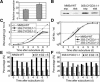
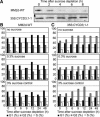
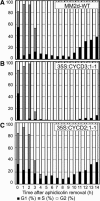
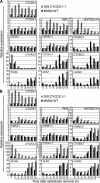
The G1-to-S-phase transition is a key regulatory point in the cell cycle, but the rate-limiting component in plants is unknown. Overexpression of CYCLIN D3;1 (CYCD3;1) in transgenic plants increases mitotic cycles and reduces endocycles, but its effects on cell cycle progression cannot be unambiguously determined. To analyze the cell cycle roles of plant D-type cyclins, we overexpressed CYCD3;1 in Arabidopsis thaliana cell suspension cultures. Changes in cell number and doubling time were insignificant, but cultures exhibited an increased proportion of G2- over G1-phase cells, as well as increased G2 arrest in response to stationary phase and sucrose starvation. Synchronized cultures confirm that CYCD3;1-expressing (but not CYCD2;1-expressing) cells show increased G2-phase length and delayed activation of mitotic genes such as B-type cyclins, suggesting that CYCD3;1 has a specific G1/S role. Analysis of putative cyclin-dependent kinase phosphorylation sites within CYCD3;1 shows that mutating Ser-343 to Ala enhances CYCD3;1 potency without affecting its rate of turnover and results in a fivefold increase in the level of cell death in response to sucrose removal. We conclude that CYCD3;1 dominantly drives the G1/S transition, and in sucrose-depleted cells the decline in CYCD3;1 levels leads to G1 arrest, which is overcome by ectopic CYCD3;1 expression. Ser-343 is likely a key residue in modulating CYCD3;1 activity in response to sucrose depletion.
Figures


Similar articles
-
Differential stability of Arabidopsis D-type cyclins: CYCD3;1 is a highly unstable protein degraded by a proteasome-dependent mechanism.Plant J. 2004 May;38(4):616-25. doi: 10.1111/j.0960-7412.2004.02071.x. Plant J. 2004. PMID: 15125768
-
Arabidopsis CYCD3 D-type cyclins link cell proliferation and endocycles and are rate-limiting for cytokinin responses.Proc Natl Acad Sci U S A. 2007 Sep 4;104(36):14537-42. doi: 10.1073/pnas.0704166104. Epub 2007 Aug 28. Proc Natl Acad Sci U S A. 2007. PMID: 17726100 Free PMC article.
-
Sugar control of the plant cell cycle: differential regulation of Arabidopsis D-type cyclin gene expression.Mol Cell Biol. 2000 Jul;20(13):4513-21. doi: 10.1128/MCB.20.13.4513-4521.2000. Mol Cell Biol. 2000. PMID: 10848578 Free PMC article.
-
Regulation of cell cycle entry and G1 progression by CSF-1.Mol Reprod Dev. 1997 Jan;46(1):11-8. doi: 10.1002/(SICI)1098-2795(199701)46:1<11::AID-MRD3>3.0.CO;2-U. Mol Reprod Dev. 1997. PMID: 8981358 Review.
-
Cytometry of cyclin proteins.Cytometry. 1996 Sep 1;25(1):1-13. doi: 10.1002/(SICI)1097-0320(19960901)25:1<1::AID-CYTO1>3.0.CO;2-N. Cytometry. 1996. PMID: 8875049 Review.
Cited by
-
Genome-Wide Identification, Characterization, and Transcriptomic Analysis of the Cyclin Gene Family in Brassica rapa.Int J Mol Sci. 2022 Nov 13;23(22):14017. doi: 10.3390/ijms232214017. Int J Mol Sci. 2022. PMID: 36430495 Free PMC article.
-
Overexpression Populus d-Type Cyclin Gene PsnCYCD1;1 Influences Cell Division and Produces Curved Leaf in Arabidopsis thaliana.Int J Mol Sci. 2021 May 29;22(11):5837. doi: 10.3390/ijms22115837. Int J Mol Sci. 2021. PMID: 34072501 Free PMC article.
-
Differential floral development and gene expression in grapevines during long and short photoperiods suggests a role for floral genes in dormancy transitioning.Plant Mol Biol. 2010 May;73(1-2):191-205. doi: 10.1007/s11103-010-9611-x. Epub 2010 Feb 12. Plant Mol Biol. 2010. PMID: 20151315
-
Are small GTPases signal hubs in sugar-mediated induction of fructan biosynthesis?PLoS One. 2009 Aug 12;4(8):e6605. doi: 10.1371/journal.pone.0006605. PLoS One. 2009. PMID: 19672308 Free PMC article.
-
The AUXIN BINDING PROTEIN 1 is required for differential auxin responses mediating root growth.PLoS One. 2009 Sep 24;4(9):e6648. doi: 10.1371/journal.pone.0006648. PLoS One. 2009. PMID: 19777056 Free PMC article.
References
-
- Araki, S., Ito, M., Soyano, T., Nishihama, R., and Machida, Y. (2004). Mitotic cyclins stimulate the activity of c-Myb-like factors for transactivation of G(2)/M-phase-specific genes in tobacco. J. Biol. Chem. 279 32979–32988. - PubMed
-
- Boonstra, J. (2003). Progression through the G(1)-phase of the on-going cell cycle. J. Cell. Biochem. 90 244–252. - PubMed
-
- Boucheron, E., Healy, J.M.S., Bajon, C., Sauvanet, A., Rembur, J., Noin, M., Sekine, M., Riou-Khamlichi, C., Murray, J.A.H., Van Onckelen, H., and Chriqui, D. (2005). Ectopic expression of Arabidopsis CycD2 and CycD3 in tobacco has distinct effects on the structural organization of the shoot apical meristem. J. Exp. Bot. 56 123–134. - PubMed
-
- Cockcroft, C.E., den Boer, B.G., Healy, J.M., and Murray, J.A.H. (2000). Cyclin D control of growth rate in plants. Nature 405 575–579. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

