Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells
- PMID: 16507771
- PMCID: PMC1895817
- DOI: 10.1182/blood-2005-08-3531
Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells
Abstract
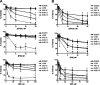
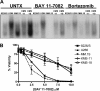
Multiple myeloma (MM) is an incurable plasma cell malignancy. The 26S proteasome inhibitor, bortezomib, selectively induces apoptosis in MM cells; however, the nature of its selectivity remains unknown. Here we demonstrate that 5 different MM cell lines display similar patterns of sensitivity to 3 proteasome inhibitors (PIs) but respond differently to specific NF-kappaB inhibition. We further show that PIs initiate the unfolded protein response (UPR), a signaling pathway activated by the accumulation of misfolded proteins within the endoplasmic reticulum (ER). Consistent with reports that prosurvival/physiologic UPR components are required for B-cell differentiation into antibody-secreting cells, we found that MM cells inherently expressed the ER chaperones GRP78/Bip and GRP94/gp96. However, bortezomib rapidly induced components of the proapoptotic/terminal UPR, including PERK, the ER stress-specific eIF-2alpha kinase; ATF4, an ER stress-induced transcription factor; and its proapoptotic target, CHOP/GADD153. Consistent with our hypothesis that PIs induce the accumulation of misfolded ER-processed proteins, we found that the amount of immunoglobulin subunits retained within MM cells correlated with their sensitivity to PIs. These findings suggest that MM cells have a lower threshold for PI-induced UPR induction and ER stress-induced apoptosis because they constitutively express ER stress survival factors to function as secretory cells.
Figures


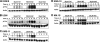

Similar articles
-
Extensive immunoglobulin production sensitizes myeloma cells for proteasome inhibition.Cancer Res. 2007 Feb 15;67(4):1783-92. doi: 10.1158/0008-5472.CAN-06-2258. Cancer Res. 2007. PMID: 17308121
-
Caspase-2 functions upstream of mitochondria in endoplasmic reticulum stress-induced apoptosis by bortezomib in human myeloma cells.Mol Cancer Ther. 2008 Aug;7(8):2298-307. doi: 10.1158/1535-7163.MCT-08-0186. Mol Cancer Ther. 2008. PMID: 18723477
-
Ritonavir induces endoplasmic reticulum stress and sensitizes sarcoma cells toward bortezomib-induced apoptosis.Mol Cancer Ther. 2008 Jul;7(7):1940-8. doi: 10.1158/1535-7163.MCT-07-2375. Mol Cancer Ther. 2008. PMID: 18645004
-
Proteasome inhibitors in the clinical setting: benefits and strategies to overcome multiple myeloma resistance to proteasome inhibitors.Drugs R D. 2007;8(1):1-12. doi: 10.2165/00126839-200708010-00001. Drugs R D. 2007. PMID: 17249845 Review.
-
The proteasome: a novel target for anticancer therapy.Clin Transl Oncol. 2006 May;8(5):313-7. doi: 10.1007/s12094-006-0176-8. Clin Transl Oncol. 2006. PMID: 16760005 Review.
Cited by
-
Amides as bioisosteres of triazole-based geranylgeranyl diphosphate synthase inhibitors.Bioorg Med Chem. 2020 Aug 15;28(16):115604. doi: 10.1016/j.bmc.2020.115604. Epub 2020 Jun 30. Bioorg Med Chem. 2020. PMID: 32690260 Free PMC article.
-
Drug resistance in multiple myeloma: Soldiers and weapons in the bone marrow niche.Front Oncol. 2022 Sep 21;12:973836. doi: 10.3389/fonc.2022.973836. eCollection 2022. Front Oncol. 2022. PMID: 36212502 Free PMC article. Review.
-
Endoplasmic reticulum and the unfolded protein response: dynamics and metabolic integration.Int Rev Cell Mol Biol. 2013;301:215-90. doi: 10.1016/B978-0-12-407704-1.00005-1. Int Rev Cell Mol Biol. 2013. PMID: 23317820 Free PMC article. Review.
-
Unraveling the regulatory role of endoplasmic-reticulum-associated degradation in tumor immunity.Crit Rev Biochem Mol Biol. 2020 Aug;55(4):322-353. doi: 10.1080/10409238.2020.1784085. Epub 2020 Jul 7. Crit Rev Biochem Mol Biol. 2020. PMID: 32633575 Free PMC article. Review.
-
Carfilzomib alters the HLA-presented peptidome of myeloma cells and impairs presentation of peptides with aromatic C-termini.Blood Cancer J. 2016 Apr 8;6(4):e411. doi: 10.1038/bcj.2016.14. Blood Cancer J. 2016. PMID: 27058226 Free PMC article.
References
-
- Anderson KC. Bortezomib therapy for myeloma. Curr Hematol Rep. 2004;3: 65. - PubMed
-
- Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA Cancer J Clin. 2000;50: 7-33. - PubMed
-
- Masdehors P, Omura S, Merle-Beral H, et al. Increased sensitivity of CLL-derived lymphocytes to apoptotic death activation by the proteasome-specific inhibitor lactacystin. Br J Haematol. 1999;105: 752-757. - PubMed
-
- Adams J, Palombella VJ, Sausville EA, et al. Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res. 1999;59: 2615-2622. - PubMed
-
- Hideshima T, Richardson P, Chauhan D, et al. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001;61: 3071-3076. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

