Binding of the streptococcal surface glycoproteins GspB and Hsa to human salivary proteins
- PMID: 16495569
- PMCID: PMC1418684
- DOI: 10.1128/IAI.74.3.1933-1940.2006
Binding of the streptococcal surface glycoproteins GspB and Hsa to human salivary proteins
Abstract
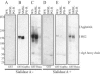
GspB and Hsa are homologous surface glycoproteins of Streptococcus gordonii that bind sialic acid moieties on platelet membrane glycoprotein Ibalpha. Since this species is an important member of the oral flora, we examined the direct binding of these adhesins to human salivary proteins. Both GspB and Hsa bound low-molecular-weight salivary mucin MG2 and salivary agglutinin. Hsa also bound several other salivary proteins, including secretory immunoglobulin A. Screening of six oral streptococcal isolates revealed that at least two of the strains expressed GspB homologues. These results indicate that GspB-like adhesins may be important for oral bacterial colonization.
Figures
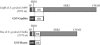




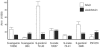

Similar articles
-
Binding of the Streptococcus gordonii surface glycoproteins GspB and Hsa to specific carbohydrate structures on platelet membrane glycoprotein Ibalpha.Mol Microbiol. 2005 Oct;58(2):380-92. doi: 10.1111/j.1365-2958.2005.04830.x. Mol Microbiol. 2005. PMID: 16194227
-
The Streptococcus gordonii surface proteins GspB and Hsa mediate binding to sialylated carbohydrate epitopes on the platelet membrane glycoprotein Ibalpha.Infect Immun. 2004 Nov;72(11):6528-37. doi: 10.1128/IAI.72.11.6528-6537.2004. Infect Immun. 2004. PMID: 15501784 Free PMC article.
-
Functions of cell surface-anchored antigen I/II family and Hsa polypeptides in interactions of Streptococcus gordonii with host receptors.Infect Immun. 2005 Oct;73(10):6629-38. doi: 10.1128/IAI.73.10.6629-6638.2005. Infect Immun. 2005. PMID: 16177339 Free PMC article.
-
Streptococcal adhesion and colonization.Crit Rev Oral Biol Med. 1997;8(2):175-200. doi: 10.1177/10454411970080020601. Crit Rev Oral Biol Med. 1997. PMID: 9167092 Review.
-
Cell surface protein receptors in oral streptococci.FEMS Microbiol Lett. 1994 Aug 15;121(2):133-40. doi: 10.1111/j.1574-6968.1994.tb07089.x. FEMS Microbiol Lett. 1994. PMID: 7926661 Review.
Cited by
-
Can salivary activity predict periodontal breakdown in A. actinomycetemcomitans infected adolescents?Arch Oral Biol. 2013 Jun;58(6):611-20. doi: 10.1016/j.archoralbio.2012.10.009. Epub 2012 Dec 6. Arch Oral Biol. 2013. PMID: 23219180 Free PMC article.
-
Streptococcus gordonii Hsa environmentally constrains competitive binding by Streptococcus sanguinis to saliva-coated hydroxyapatite.J Bacteriol. 2007 Apr;189(8):3106-14. doi: 10.1128/JB.01535-06. Epub 2007 Feb 2. J Bacteriol. 2007. PMID: 17277052 Free PMC article.
-
Streptococcus mitis phage-encoded adhesins mediate attachment to {alpha}2-8-linked sialic acid residues on platelet membrane gangliosides.Infect Immun. 2009 Aug;77(8):3485-90. doi: 10.1128/IAI.01573-08. Epub 2009 Jun 8. Infect Immun. 2009. PMID: 19506011 Free PMC article.
-
Role of Neuraminidase-Producing Bacteria in Exposing Cryptic Carbohydrate Receptors for Streptococcus gordonii Adherence.Infect Immun. 2018 Jun 21;86(7):e00068-18. doi: 10.1128/IAI.00068-18. Print 2018 Jul. Infect Immun. 2018. PMID: 29661931 Free PMC article.
-
Implications of salivary protein binding to commensal and pathogenic bacteria.J Oral Biosci. 2013 Nov 1;55(4):169-174. doi: 10.1016/j.job.2013.06.004. J Oral Biosci. 2013. PMID: 24707190 Free PMC article.
References
-
- Becerra, L., R. V. Soares, L. S. Bruno, C. C. Siqueira, F. G. Oppenheim, G. D. Offner, and R. F. Troxler. 2003. Patterns of secretion of mucins and non-mucin glycoproteins in human submandibular/sublingual secretion. Arch. Oral Biol. 48:147-154. - PubMed
-
- Bensing, B. A., D. Takamatsu, and P. M. Sullam. 2005. Determinants of the streptococcal surface glycoprotein GspB that facilitate export by the accessory Sec system. Mol. Microbiol. 58:1468-1481. - PubMed
-
- Bensing, B. A., and P. M. Sullam. 2002. An accessory sec locus of Streptococcus gordonii is required for export of the surface protein GspB and for normal levels of binding to human platelets. Mol. Microbiol. 44:1081-1094. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

