Proteomic analysis of adaptor protein 1A coats selectively assembled on liposomes
- PMID: 16492770
- PMCID: PMC1413908
- DOI: 10.1073/pnas.0511062103
Proteomic analysis of adaptor protein 1A coats selectively assembled on liposomes
Abstract
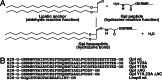
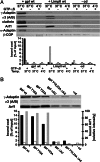
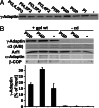
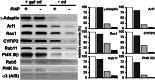
Coat components localize to specific membrane domains, where they sort selected transmembrane proteins. To study how clathrin coats are stabilized on such domains and to identify the protein networks involved, we combined proteomic screens and in vitro liposome-based assays that recapitulate the fidelity of protein sorting in vivo. Our study identifying approximately 40 proteins on AP-1A-coated liposomes revealed that AP-1A coat assembly triggers the concomitant recruitment of Rac1, its effectors, and the Wave/Scar complex as well as that of Rab11 and Rab14. The coordinated recruitment of these different machineries requires a mosaic of membrane components comprising the GTPase ADP-ribosylation factor 1, sorting signals in selected transmembrane proteins, and phosphatidylinositol 4-phosphate. These results demonstrate that the combinatorial use of low-affinity binding sites present on the same membrane domain accounts not only for a selective coat assembly but also for the coordinated assembly of selected machineries required for actin polymerization and subsequent membrane fusion.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures

Similar articles
-
Interaction of amphiphysins with AP-1 clathrin adaptors at the membrane.Biochem J. 2013 Feb 15;450(1):73-83. doi: 10.1042/BJ20121373. Biochem J. 2013. PMID: 23190214
-
Protein complexes containing CYFIP/Sra/PIR121 coordinate Arf1 and Rac1 signalling during clathrin-AP-1-coated carrier biogenesis at the TGN.Nat Cell Biol. 2010 Apr;12(4):330-40. doi: 10.1038/ncb2034. Epub 2010 Mar 14. Nat Cell Biol. 2010. PMID: 20228810 Free PMC article.
-
Protein networks supporting AP-3 function in targeting lysosomal membrane proteins.Mol Biol Cell. 2008 May;19(5):1942-51. doi: 10.1091/mbc.e08-02-0110. Epub 2008 Feb 20. Mol Biol Cell. 2008. PMID: 18287518 Free PMC article.
-
Recruitment of coat proteins to liposomes and peptidoliposomes.Methods Mol Biol. 2015;1270:91-106. doi: 10.1007/978-1-4939-2309-0_7. Methods Mol Biol. 2015. PMID: 25702111
-
Membrane recruitment of effector proteins by Arf and Rab GTPases.Curr Opin Struct Biol. 2005 Dec;15(6):681-9. doi: 10.1016/j.sbi.2005.10.015. Epub 2005 Nov 9. Curr Opin Struct Biol. 2005. PMID: 16289847 Review.
Cited by
-
A novel role for retromer in the control of epithelial cell polarity.Commun Integr Biol. 2011 Nov 1;4(6):749-51. doi: 10.4161/cib.17658. Commun Integr Biol. 2011. PMID: 22446545 Free PMC article.
-
Arf GTPase activates the WAVE regulatory complex through a distinct binding site.Sci Adv. 2022 Dec 14;8(50):eadd1412. doi: 10.1126/sciadv.add1412. Epub 2022 Dec 14. Sci Adv. 2022. PMID: 36516255 Free PMC article.
-
APP controls the formation of PI(3,5)P(2) vesicles through its binding of the PIKfyve complex.Cell Mol Life Sci. 2016 Jan;73(2):393-408. doi: 10.1007/s00018-015-1993-0. Epub 2015 Jul 28. Cell Mol Life Sci. 2016. PMID: 26216398 Free PMC article.
-
Golgi export of the Kir2.1 channel is driven by a trafficking signal located within its tertiary structure.Cell. 2011 Jun 24;145(7):1102-15. doi: 10.1016/j.cell.2011.06.007. Cell. 2011. PMID: 21703452 Free PMC article.
-
Type I gamma phosphatidylinositol phosphate kinase modulates adherens junction and E-cadherin trafficking via a direct interaction with mu 1B adaptin.J Cell Biol. 2007 Jan 29;176(3):343-53. doi: 10.1083/jcb.200606023. J Cell Biol. 2007. PMID: 17261850 Free PMC article.
References
-
- Kirchhausen T. Nat. Rev. Mol. Cell Biol. 2000;1:187–198. - PubMed
-
- Antonny B., Schekman R. Curr. Opin. Cell Biol. 2001;13:438–443. - PubMed
-
- Bonifacino J. S., Traub L. M. Annu. Rev. Biochem. 2003;72:395–447. - PubMed
-
- Owen D. J., Collins B. M., Evans P. R. Annu. Rev. Cell Dev. Biol. 2004;20:153–191. - PubMed
-
- Robinson M. S. Trends Cell Biol. 2004;14:167–174. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

