Nature of nonfunctional envelope proteins on the surface of human immunodeficiency virus type 1
- PMID: 16474158
- PMCID: PMC1395414
- DOI: 10.1128/JVI.80.5.2515-2528.2006
Nature of nonfunctional envelope proteins on the surface of human immunodeficiency virus type 1
Abstract
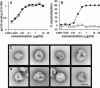
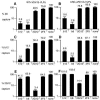
Human immunodeficiency virus type 1 (HIV-1) neutralizing antibodies are thought be distinguished from nonneutralizing antibodies by their ability to recognize functional gp120/gp41 envelope glycoprotein (Env) trimers. The antibody responses induced by natural HIV-1 infection or by vaccine candidates tested to date consist largely of nonneutralizing antibodies. One might have expected a more vigorous neutralizing response, particularly against virus particles that bear functional trimers. The recent surprising observation that nonneutralizing antibodies can specifically capture HIV-1 may provide a clue relating to this paradox. Specifically, it was suggested that forms of Env, to which nonneutralizing antibodies can bind, exist on virus surfaces. Here, we present evidence that HIV-1 particles bear nonfunctional gp120/gp41 monomers and gp120-depleted gp41 stumps. Using a native electrophoresis band shift assay, we show that antibody-trimer binding predicts neutralization and that the nonfunctional forms of Env may account for virus capture by nonneutralizing antibodies. We hypothesize that these nonfunctional forms of Env on particle surfaces serve to divert the antibody response, helping the virus to evade neutralization.
Figures



Similar articles
-
Discrete partitioning of HIV-1 Env forms revealed by viral capture.Retrovirology. 2015 Sep 24;12:81. doi: 10.1186/s12977-015-0207-z. Retrovirology. 2015. PMID: 26399966 Free PMC article.
-
HIV type 1 Env precursor cleavage state affects recognition by both neutralizing and nonneutralizing gp41 antibodies.AIDS Res Hum Retroviruses. 2011 Aug;27(8):877-87. doi: 10.1089/AID.2010.0281. Epub 2011 Jan 19. AIDS Res Hum Retroviruses. 2011. PMID: 21158699 Free PMC article.
-
A comparative immunogenicity study of HIV-1 virus-like particles bearing various forms of envelope proteins, particles bearing no envelope and soluble monomeric gp120.Virology. 2007 Sep 30;366(2):245-62. doi: 10.1016/j.virol.2007.04.033. Epub 2007 Jun 19. Virology. 2007. PMID: 17580087 Free PMC article.
-
Relevance of the antibody response against human immunodeficiency virus type 1 envelope to vaccine design.Immunol Lett. 1997 Jun 1;57(1-3):105-12. doi: 10.1016/s0165-2478(97)00043-6. Immunol Lett. 1997. Corrected and republished in: Immunol Lett. 1997 Jul;58(2):125-32. doi: 10.1016/s0165-2478(97)00109-0 PMID: 9232434 Corrected and republished. Review.
-
Erratum to "Relevance of the antibody response against human immunodeficiency virus type 1 envelope to vaccine design".Immunol Lett. 1997 Jul;58(2):125-32. doi: 10.1016/s0165-2478(97)00109-0. Immunol Lett. 1997. PMID: 9271324 Review.
Cited by
-
A Blueprint for HIV Vaccine Discovery.Cell Host Microbe. 2012 Oct 18;12(4):396-407. doi: 10.1016/j.chom.2012.09.008. Cell Host Microbe. 2012. PMID: 23084910 Free PMC article. Review.
-
Neutralization of tier-2 viruses and epitope profiling of plasma antibodies from human immunodeficiency virus type 1 infected donors from India.PLoS One. 2012;7(8):e43704. doi: 10.1371/journal.pone.0043704. Epub 2012 Aug 31. PLoS One. 2012. PMID: 22952740 Free PMC article.
-
Common helical V1V2 conformations of HIV-1 Envelope expose the α4β7 binding site on intact virions.Nat Commun. 2018 Oct 26;9(1):4489. doi: 10.1038/s41467-018-06794-x. Nat Commun. 2018. PMID: 30367034 Free PMC article.
-
Conformational plasticity of the HIV-1 gp41 immunodominant region is recognized by multiple non-neutralizing antibodies.Commun Biol. 2022 Mar 31;5(1):291. doi: 10.1038/s42003-022-03235-w. Commun Biol. 2022. PMID: 35361878 Free PMC article.
-
Effect of trimerization motifs on quaternary structure, antigenicity, and immunogenicity of a noncleavable HIV-1 gp140 envelope glycoprotein.Virology. 2009 Dec 5;395(1):33-44. doi: 10.1016/j.virol.2009.07.042. Epub 2009 Oct 8. Virology. 2009. PMID: 19815247 Free PMC article.
References
-
- Abacioglu, Y. H., T. R. Fouts, J. D. Laman, E. Claassen, S. H. Pincus, J. P. Moore, C. A. Roby, R. Kamin-Lewis, and G. K. Lewis. 1994. Epitope mapping and topology of baculovirus-expressed HIV-1 gp160 determined with a panel of murine monoclonal antibodies. AIDS Res. Hum. Retroviruses 10:371-381. - PubMed
-
- Allaway, G. P., K. L. Davis-Bruno, G. A. Beaudry, E. B. Garcia, E. L. Wong, A. M. Ryder, K. W. Hasel, M. C. Gauduin, R. A. Koup, J. S. McDougal, et al. 1995. Expression and characterization of CD4-IgG2, a novel heterotetramer that neutralizes primary HIV type 1 isolates. AIDS Res. Hum. Retrovir. 11:533-539. - PubMed
-
- Bewley, C. A., J. M. Louis, R. Ghirlando, and G. M. Clore. 2002. Design of a novel peptide inhibitor of HIV fusion that disrupts the internal trimeric coiled-coil of gp41. J. Biol. Chem. 277:14238-14245. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

